Pasteurisation of liquid whole egg with pulsed electric fields
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 7 July 2011 | Silvia Monfort, Santiago Condón, Javier Raso & Ignacio Álvarez Tecnología de los Alimentos, Facultad de Veterinaria, Universidad de Zaragoza | No comments yet
In literature, there are many publications related to the microbial inactivation by PEF in LWE. However, there is not yet an answer to the question: is it possible to pasteurise liquid whole egg with pulsed electric fields? This could be due to the difficulties in comparing results since different treatment conditions have been used by authors, the narrow range of microbial species investigated, or the study of PEF lethality by the end-point method, among other reasons. This article summarises the results obtained by the group ‘New technologies of food preservation’ at the University of Zaragoza (Spain) carried out in order to answer that question.
Eggs and egg products are a nutritious part of our diet and a useful ingredient in foods due to their functional properties. Unfortunately, they are responsible for a large number of foodborne illnesses each year, constituting an obstacle to the well-being of populations and a source of high economic losses. In 2006, from a total of 5,710 outbreaks in Europe, egg and egg products were the most common food vehicle, responsible for 11.7 per cent of these outbreaks. Salmonella – mainly serovars Salmonella Enteritidis and Salmonella Typhimurium – was the microorganism responsible in most of the cases1.
In literature, there are many publications related to the microbial inactivation by PEF in LWE. However, there is not yet an answer to the question: is it possible to pasteurise liquid whole egg with pulsed electric fields? This could be due to the difficulties in comparing results since different treatment conditions have been used by authors, the narrow range of microbial species investigated, or the study of PEF lethality by the end-point method, among other reasons. This article summarises the results obtained by the group ‘New technologies of food preservation’ at the University of Zaragoza (Spain) carried out in order to answer that question. Eggs and egg products are a nutritious part of our diet and a useful ingredient in foods due to their functional properties. Unfortunately, they are responsible for a large number of foodborne illnesses each year, constituting an obstacle to the well-being of populations and a source of high economic losses. In 2006, from a total of 5,710 outbreaks in Europe, egg and egg products were the most common food vehicle, responsible for 11.7 per cent of these outbreaks. Salmonella – mainly serovars Salmonella Enteritidis and Salmonella Typhimurium – was the microorganism responsible in most of the cases1.
In literature, there are many publications related to the microbial inactivation by PEF in LWE. However, there is not yet an answer to the question: is it possible to pasteurise liquid whole egg with pulsed electric fields? This could be due to the difficulties in comparing results since different treatment conditions have been used by authors, the narrow range of microbial species investigated, or the study of PEF lethality by the end-point method, among other reasons. This article summarises the results obtained by the group ‘New technologies of food preservation’ at the University of Zaragoza (Spain) carried out in order to answer that question.
Eggs and egg products are a nutritious part of our diet and a useful ingredient in foods due to their functional properties. Unfortunately, they are responsible for a large number of foodborne illnesses each year, constituting an obstacle to the well-being of populations and a source of high economic losses. In 2006, from a total of 5,710 outbreaks in Europe, egg and egg products were the most common food vehicle, responsible for 11.7 per cent of these outbreaks. Salmonella – mainly serovars Salmonella Enteritidis and Salmonella Typhimurium – was the microorganism responsible in most of the cases1.
European Regulation CEE 1441/20072 requires the absence of Salmonella in 25 grams or mL of liquid whole egg (LWE) to offer a safe product, and in the United States, at least five Log10 cycles of inactivation of any Salmonella species are demanded3. Currently, the egg industry’s primary intervention to improve the microbiological safety of liquid egg is thermal treatment (low intensity treatments of 60°C/3.5’ or 64°C/2.5’; or high intensity ultra pasteurisation at 70 – 71°C/1.5’). However, the thermal sensitivity of liquid egg components limits the temperature at which the product can be heated; some soluble proteins begin to precipitate at temperatures as low as 57°C4,5. The temperatures required for pasteurisation of LWE can induce changes in egg quality, including coagulation of egg proteins and changes in other functional properties. Therefore, new non thermal technologies are being investigated to inactivate Salmonella6,7 in LWE with a minimum impact on the freshness properties8-11.
Pulsed electric fields (PEF) technology is evaluated as a possible technology to hygienise LWE. It has been observed in other products that PEF can inactivate vegetative cells of spoiling and pathogenic bacteria at ambient temperature, diminishing consequently the negative impact of heat on the quality properties12. The process involves the application of short duration pulses of high electric field strengths (1-50 kV/cm) to foods placed between two electrodes. These field strengths create pores on cell membranes (electroporation) leading to cellular death without affecting other cellular structures or food components such as proteins13.
Although a great effort has been made to evaluate the actual possibilities of PEF in the food industry in recent years, there are still many unanswered questions, mainly about the hygienisation of LWE. PEF technology has been demonstrated as effective in inactivating various serotypes of Salmonella, including Enteritidis, Dublin, Typhimurium, and Senftenberg, when inoculated in foods other than LWE9,14-19. Studies on microbial inactivation by PEF in LWE have been published20-24, but most studies used bacteria other than Salmonella spp. The few investigations related to PEF inactivation of Salmonella serotypes in LWE have been carried out with only one serotype, using the end point method, or applying PEF treatments in combination with other preservation technologies9,15,25-29. Thus, it is difficult to properly evaluate the viability of PEF as a LWE hygienisation system.
On the other hand, one important factor that has to be considered when using PEF technology for industrial application is that in a continuous regimen, all the PEF energy applied in the treatment chamber where the product is treated turns into heat due to the Joule effect, increasing the temperature of the product. This is relevant in LWE due to its high electrical conductivity (around 7 mS/cm at 24°C), which involves a great discharge of energy per pulse applied, and its thermal-sensitivity quality properties, which decrease at temperatures over 57°C and LWE completely coagulates at 73°C. Considering this point, a threshold energy of 250 kJ/kg, assuming a corresponding warming of approximately 65°C, could be considered30. For this specific energy level, and based on data published in literature, around two Log10 cycles of inactivation of Salmonella would be achieved by PEF treatments in LWE, shown in Figure 1 (i.e., 43 kV/cm and 20 μs – red line – or 26 kV/cm and 60 μs – blue line). This figure was obtained with mathematical models (Table 1) developed from survival curves obtained for Salmonella serovars Enteritidis, Typhimurium, and Senftenberg 775W in LWE in a wide range of PEF treatment conditions (20-45 kV/cm, 0-150 μs, 0-500 kJ/kg) at temperatures lower than 35°C31,32. From these results, it can be concluded that the level of inactivation achieved with this maximum PEF energy is far from the 5 Log10 cycles of inactivation of Salmonella recommended by the USDA and could be insufficient to guarantee the absence of Salmonella in 25 grams or mL of LWE indicated by European Regulation. Therefore, PEF technology by itself is not suitable for pasteurising LWE.
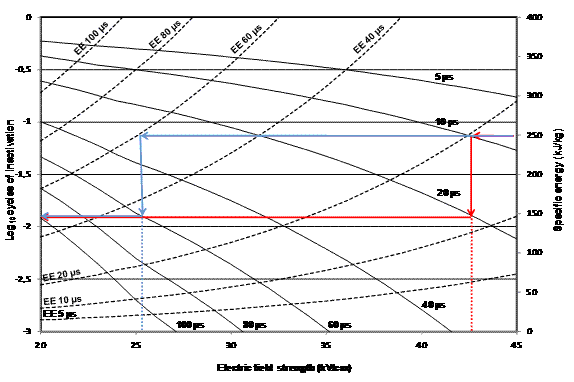

Figure 1 Combinations of electric field strength, treatment time, and specific energy needed to achieve a certain level of inactivation of Salmonella serovars Enteritidis, Typhimurium and Senftenberg 775W treated by PEF in LWE. Continuous lines indicate the Log10 cycles of inactivation of Salmonella (left OY axis), achieved at different treatment times and electric field strengths. The dotted lines (EE – energetic equivalent) correspond to the specific energy required (right OY axis) to achieve the same level of inactivation than the corresponding treatment time at each electric field strength. Blue and red lines indicate examples of the treatment time, field strength and inactivation achieved with an energy level of 250 kJ/kg.
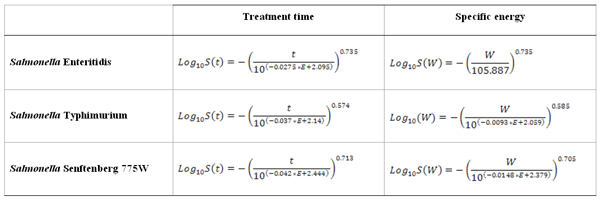

Table 1 Mathematical models developed to predict Salmonella Enteritidis, Typhimurium and Salmonella Senftenberg 775W inactivation by PEF in LWE in terms of the treatment time and the specific energy (E is the electric field strength expressed in kV/cm; t is the treatment time in μs; Wis the specific energy in kJ/kg).
To increase the viability of PEF technology as a LWE pasteurisation system, it is necessary to improve the lethal effectiveness of PEF by combining it with other preservation technologies. The combination of PEF with antimicrobials or with temperature or the successive application of PEF and heat has been evaluated in LWE or egg whites22,25,26,28. For LWE, the lethal effect of PEF in combination with antimicrobials (nisin, citric acid, and CocoanOX) has been shown to be effective (up to 5 Log10 cycles) to inactivate Gram positive bacteria (Listeria innocua, Micrococcus luteus, and Bacillus cereus)22,33-35. Results obtained in our research group indicated that the application of PEF treatments to LWE in the presence of up to eight different additives has permitted a total reduction of approximately three Log10 in the population of Salmonella Enteritidis32. On the other hand, the application of PEF at temperatures up to 60°C, PEF followed by heat or heat followed by PEF treatments in LWE or egg products inactivated four Log10 cycles in LWE and five Log10 cycles in egg yolks for Salmonella Enteritidis or E. coli 0157:H7, respectively15,26. Although additive and synergistic lethal effects have been observed, the achieved levels of inactivation with the combined processes described in literature could be insufficient to obtain Salmonella-free LWE.
Recently, Monfort et al.32 designed a combined process based on the application of a successive treatment of PEF followed by heat in the presence of triethyl citrate (one or two per cent). The application of a previous PEF treatment (25 kV/cm and 100 kJ/kg) in the presence of this additive (E1505), which can be added to egg products to improve their whipping properties, reduced the heat resistance to a posterior thermal treatment of Salmonella serovars up to 100 fold, permitting more than 8-Log10 reductions with heat treatments of 52°C/3.5’, 55 °C/2.5’ or 60°C/1.5’ (Figure 2). These combined treatments are also effective against other Salmonella serovars (Table 2). As observed in Table 2, the level of inactivation achieved is similar or even higher than traditional low intensity heat pasteurisation treatments of 60°C/3.5’(or 64°C/2.5’ – data not shown). For the high intensity ultra pasteurisation process at 70 – 71°C/1.5’, an alternative treatment has been proposed with similar Salmonella lethality: PEF (25 kV/cm and 100 kJ/kg) followed by a heat treatment of 60°C/3.5’ in the presence of one per cent triethyl citrate (TC) (Table 2). These results indicate that designed treatments could be alternatives to current heat pasteurisation treatments of LWE at both levels, low and high intensity pasteurisation.
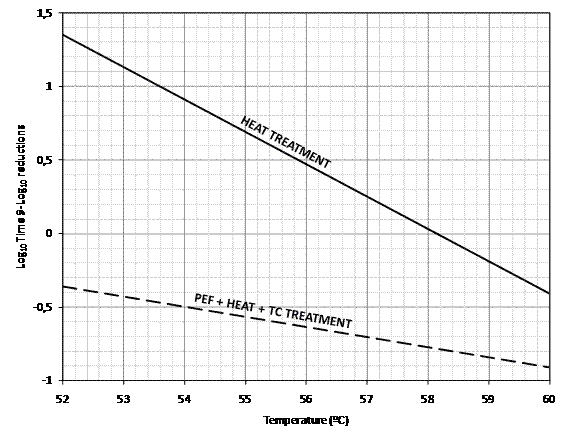

Figure 2TDT lines for 9- Log10 reductions of Salmonella Enteritidis treated by heat (continuous line) or PEF (25 kV/cm; 100 kJ/Kg) followed by heat in presence of two per cent triethyl citrate (dotted line) in LWE
Table 2: Log reductions of different Salmonella serovars treated by heat or by PEF followed by heat treatments in presence of triethyl citrate in LWE
|
|
Low pasteurization |
High pasteurization |
||
| Salmonella serovar |
PEF + 52ºC/3.5′ + 2% TC |
60ºC/3.5′ |
PEF + 60ºC/3.5′ + 1% TC |
71ºC/1.5′ |
| Dublin |
> 8.0 |
4.3 |
> 8.0 |
> 8.0 |
| Enteritidis |
7.7 |
7.4 |
> 8.0 |
> 8.0 |
| Senftenberg |
5.5 |
2.0 |
> 8.0 |
> 8.0 |
| Typhi |
> 8.0 |
> 8.0 |
> 8.0 |
> 8.0 |
| Typhimurium |
6.9 |
> 8.0 |
> 8.0 |
> 8.0 |
| Virchow |
> 8.0 |
> 8.0 |
> 8.0 |
> 8.0 |
From a technological standpoint, the reduction in temperature and heating time (Figure 3) would be beneficial to maintain the functional properties of treated LWE similar to those of non-treated LWE. The evaluation of the impact of the treatments on the functional properties of LWE indicated that the quality of LWE, in terms of soluble protein, foaming and emulsify capacity, processed by the designed treatments at both levels (low and high intensities), was better than the LWE treated by comparable traditional heat pasteurisation treatments (Table 3). Moreover, the high intensity PEF-based combined process at 60°C/3.5’ showed even better foaming and emulsifying properties than the traditional low intensity treatment at 60°C/3.5’ or than the non-treated LWE.
Table 3: Physico-chemical and functional properties of LWE treated by the designed combined processes and current heat pasteurisation treatments. Numbers are expressed in percentage (%) with respect to non-treated LWE
|
|
Low pasteurization |
High pasteurization |
||
|
|
PEF + 52ºC/3.5’+ 2% TC |
60ºC/3.5’ |
PEF + 60ºC/3.5’ + 1% TC |
71ºC/1.5’ |
| Soluble protein |
99.0 |
96.6 |
99.8 |
76.9 |
| Viscosity |
113.8 |
118.8 |
154.1 |
257.0 |
| Foaming capacity |
130.0 |
73.0 |
111.7 |
36.0 |
| Gelling capacity |
98.4 |
103.2 |
109.1 |
94.8 |
| Emulsify capacity |
109.0 |
66.1 |
104.20 |
31.0 |
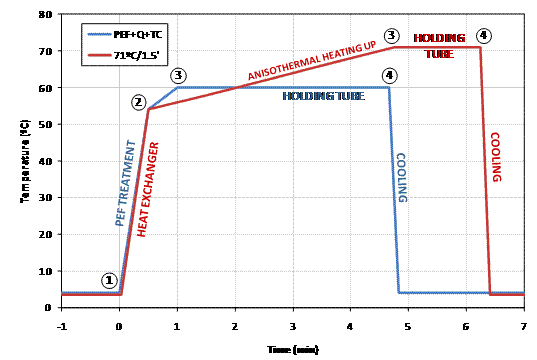

Figure 3 Figure 3: Schematic comparison of the industrial ultra pasteurization treatment (red line) and the PEF followed by heat treatment in presence of TC – PEF+Q+TC – (blue line) of LWE. Stages:1Heating-up phase: in a heat exchanger for ultra pasteurisation and in the PEF treatment chamber for PEF+Q+TC; 2 Homogenisation; 2-3 An isothermal heating-up; 3 Heat treatment in holding tube; 4 Cooling in heat exchanger.
In conclusion, results in literature indicate that the lethal effectiveness of PEF treatments at temperatures lower than 35°C is not sufficient to obtain Salmonella-free LWE and therefore is insufficient to pasteurise LWE. Thus, it is necessary to combine PEF treatments with other strategies, becoming the application of PEF treatments followed by heat in the presence of additives an alternative to current heat pasteurisation treatments. The application of a PEF treatment of 25 kV/cm and 100 kJ/kg followed by heat treatments of 52 °C/3.5’ in the presence of two per cent TC or 60°C/3.5’ in presence of one per cent TC could be promising alternatives to low (60°C/3.5’ or 64°C/2.5’) and high (71°C/1.5’) pasteurisation heat treatments, respectively. These new design treatments permit similar levels of safety in LWE with better quality properties in terms of foaming and emulsifying capacity based on shorter and lower treatment times and temperatures.
Acknowledgements
The authors thank CICYT (project AGL 2007- 62738) for the support. S.M. gratefully acknowledges the financial support for her doctoral studies from the Department of Science, Technology and University of the Aragon.
References
1. European Food Safety Authority & European Centre for Diseases and Control, 2009. The community summary report on trends and sources of zoonoses and zoonotic agents in the European union in 2007. Available from: EFSA J. 223 http:// www.efsa.europa.eu/EFSA/ efsa_locale-1178620753812_1211902269834.htm (Accessed 30.5.10)
2. Commission Regulation (EC) No. 1441/2007 of 5 December 2007 amending Regulation (EC) No. 2073/2005 on microbiological criteria for foodstuffs. Official Journal of the European Union 7.12.2007, L 322/12-L 322/29
3. USDA, 1969. Egg Pasteurization Manual. ARS 74–48. Poultry Laboratory, Agricultural Research Service, U.S. Department of Agriculture, Albany, CA.
4. Hamid-Samimi, M., Swartzel, K.R., Ball, H.R. 1984. Flow behaviour of liquid whole egg during thermal treatments. Journal of Food Science 49: 132-136
5. Herald, T.J., Smith, D.M. 1989. Functional properties and composition of liquid whole egg proteins as influenced by pasteurization and frozen storage. Poultry Science 68: 1461-1469
6. Erickson, J. P., & Jenkins, P. 1992. Behaviour of psychrotropic pathogens Listeria monocytogenes, Yersinia enterocolitica and Aeromonas hydrophila in commercially pasterurized eggs held at 2, 6.7 and 12.8°C. Journal of Food Protection 55: 8−12
7. Foegeding, P. M., & Stanley, N. W. 1987. Growth and inactivation of microorganisms isolated from ultrapasteurized egg. Journal of Food Protection 52(5): 1219−1227
8. Álvarez, I., Niemira, B.A., Fan, X., Sommers, C.H. 2006. Inactivation of Salmonella serovars in liquid whole egg by heat following irradiation treatments. Journal of Food Protection 69 (9): 2066–2074
9. Jeantet, R., Baron, F., Nau, F., Roignant, M., Brulé, G. 1999. High intensity pulsed electric fields applied to egg white: effect on Salmonella enteritidis inactivation and protein denaturation. Journal of Food Protection 62: 1381-1386
10. Lee, D. U., Heinz, V., Knorr, D. 2003. Effects of combination treatments of nisin and high-intensity ultrasound with high pressure on the microbial inactivation in liquid whole egg. Innovative Food Science and Emerging Technologies 4: 387−393
11. Mañas, P., Pagán, R., Álvarez, I., Condón, S. 2003. Survival of Salmonella senftenberg 775 W to current liquid whole egg pasteurization treatments. Food Microbiology 20: 593-600
12. Barbosa-Cánovas, G.V., Pierson, M.D., Zhang, Q.H., Schaffner, D.W. 2001. Pulsed electric fields. Journal of Food Science 65–79 Supplement
13. Wouters, P., Álvarez, I., Raso, J. 2001. Critical factors determining inactivation kinetics by pulsed electric field food processing. Trends Food Science and Technology 12: 112−121
14. Álvarez, I., Manas, P., Condón, S., Sala, F.J., Raso, J. 2003. Resistance variation of Salmonella enterica serovars to pulsed electric fields treatments. Journal of Food Science 68: 2316-2320
15. Amiali, M., Ngadi, M.O., Smith, J.P., Raghavan, G.S.V. 2006. Inactivation of Escherichia coli O157:H7 and Salmonella enteritidis in liquid egg white using pulsed electric field. Journal of Food Science 71: 88-94
16. Liang, Z., Mittal, G.S., Griffiths, M.W. 2002. Inactivation of Salmonella typhimurium in orange juice containing antimicrobial agents by pulsed electric fields. Journal of Food Protection 65: 1081-1087
17. Mosqueda-Melgar, J., Raybaudi-Massilia, R.M., Martin- Belloso, O., 2007. Influence of treatment time and pulse frequency on Salmonella Enteritidis, Escherichia coli and Listeria monocytogenes populations inoculated in melon and watermelon juices treated by pulsed electric fields. International Journal of Food Microbiology 117: 192-200
18. Raso, J., Álvarez, I., Condón, S., Sala, F.J. 2000. Predicting inactivation of Salmonella senftenberg by pulsed electric fields. Innovative Food Science and Emerging Technologies 1: 21–30
19. Sensoy, I., Zhang, Q.H., Sastry, S.K. 1997. Inactivation kinetics of Salmonella dublin by pulsed electric field. Journal of Food Process and Engineering. 20, 367-381
20. Amiali, M., Ngadi, M.O., Raghavan, V.G.S., Smith, J.P. 2004. Inactivation of Escherichia coli O157:H7 in liquid dialyzed egg using pulsed electric fields. Food Bioproduct Process 82: 151-156
21. Bazhal, M.I., Ngadi, M.O., Raghavan, G.S.V., Smith, J.P. 2006. Inactivation of Escherichia coli O157:H7 in liquid whole egg using combined pulsed electric field and thermal treatments. LWT Food Science and Technology 39: 419-425
22. Calderon-Miranda, M.L., Barbosa-Canovas, G.V., Swanson, B.G. 1999. Inactivation of Listeria innocua in liquid whole egg by pulsed electric fields and nisin. International Journal of Food Microbiology 51:7–17
23. Góngora-Nieto, M.M., Seignour, L., Riquet, P., Davidson, P.M., Barbosa-Cánovas, G.V., Swanson, B.G. 2001. Nonthermal inactivation of Pseudomonas fluorescens in liquid whole egg. In: Barbosa-Cánovas, G.V., Zhang, H. (Eds.), Pulsed Electric Fields in Food Processing: Fundamental Aspects and Applications. Technomic Publishing, Lancaster, PA, pp. 193-210
24. Martin-Belloso, O., Vega-Mercado, H., Qin, B.L., Chang, F.J., Barbosa-Canovas, G.V., Swanson, B.G. 1997. Inactivation of Escherichia coli suspended in liquid egg using pulsed electric fields. Journal of Food Processing and Preservation 21: 193-208
25. Amiali, M., Ngadi, M.O., Smith, J.P., Raghavan, G.S.V. 2007. Synergistic effect of temperature and pulsed electric field on inactivation of Escherichia coli O157:H7 and Salmonella enteritidis in liquid egg yolk. Journal of Food Engineering 79: 689-694
26. Hermawan, N., Evrendilek, G.A., Dantzer, W.R., Zhang, Q.H., Richter, E.R. 2004. Pulsed electric field treatment of liquid whole egg inoculated with Salmonella Enteritidis. Journal of Food Safety 24: 71-85
27. Huang, E., Mittal, G.S., Griffiths, M.W. 2006. Inactivation of Salmonella enteritidis in liquid whole egg using combination treatments of pulsed electric field, high pressure and ultrasound. Biosystems Engineering 94: 403-413
28. Jin, T., Zhang, H., Hermawan, N., Dantzer, W. 2009. Effects of pH and temperature on inactivation of Salmonella typhimurium DT104 in liquid whole egg by pulsed electric fields. International Journal of Food Science and Technology 44: 367-372
29. Monfort, S., Gayán, E., Saldaña, G., Puértolas, E., Condón, S., Raso, J., Álvarez, I. 2010b. Inactivation of Salmonella Typhimurium and Staphylococcus aureus by pulsed electric fields in liquid whole egg. Innovative Food Science and Emerging Technologies 11: 306-313
30. Heinz, V., Álvarez, I., Angersbach, A., Knorr, D. 2002. Preservation of liquid foods by high intensity pulsed electric fields e basic concepts for process design. Trends Food Science and Technology 12: 103-111
31. Monfort, S., Gayán, E., Raso, J., Condón, S., Álvarez, I. 2010a. Evaluation of pulsed electric fields technology for liquid whole egg pasteurization. Food Microbiology 27 (7): 845–852
32. Monfort, S., Gayán, E., Condón, S., Raso, J., Álvarez, I. 2011. Design of a combined process for the inactivation of Salmonella Enteritidis in liquid whole egg at 55°C. International Journal of Food Microbiology 145: 476–482
33. Dutreux, N., Notermans, S., GongoraNieto, M.M., BarbosaCanovas, G.V., Swanson, B.G. 2000. Effects of combined exposure of Micrococcus luteus to nisin and pulsed electric fields. International Journal of Food Microbiology 60: 147–152
34. Góngora-Nieto, M.M., Pedrow, P.D., Swanson, B.G., Barbosa-Cánovas, G.V. 2003. Energy analysis of liquid whole egg pasteurized by pulsed electric fields. Journal of Food Engineering 57: 209–216
35. Pina-Perez, M.C., Silva-Angulo, A.B., Rodrigo, D., Martinez- Lopez, A. 2009. Synergistic effect of pulsed electric fields and CocoanOX 12 per cent on the inactivation kinetics of Bacillus cereus in a mixed beverage of liquid whole egg and skim milk. International Journal of Food Microbiology 130: 196–204
About the Authors
Silvia Monfort has a degree in Chemical Engineering and a Master’s degree in Food Science and Technology from the University of Zaragoza. In 2007, she obtained a fellowship at the National Institute for Agricultural Technology in Buenos Aires, and in 2008, at the Laboratory of Food Technology at Katholieke Universiteit Leuven. Currently, Silvia is working as PhD student at the Laboratory of New Technologies for Food Preservation, University of Zaragoza, performing research on pasteurisation of liquid whole egg (LWE) by combined processes based on pulsed electric fields. In 2010, she has been a visiting researcher scholar at TU Berlin evaluating the possibilities of high hydrostatic pressure treatments for LWE pasteurisation.
Dr. Javier Raso is currently professor of Food Technology at the University of Zaragoza. He has been visiting researcher at Unilever Research in Bedford, at TU Berlin and at Washington State University. His areas of research are in the field of food preservation by techniques that inactivate microorganisms such as heat, ultrasound, high hydrostatic pressures, pulsed electric fields and combined processing. He has been involved in a number of EU and national funded projects in these topics and has published more that 50 peer-review papers.
Dr. Santiago Condón is professor of Food Technology at the University of Zaragoza and head of the New Technologies for Food Preservation. He has received different awards: 3M foundation Award (2001), Enterprise IDEA Award 2007, Coris Gruart Award (2007), Foods of Aragón Award 2011, among others. He has published more than 100 scientific publications. Among his more excellent contributions has been to emphasise the development of manothermosonication and manosonication processes, two biological tests for detection of antibiotics in milk and meat, and the design of the thermorresistometer TRSC used in labs of all around the word.
Dr. Ignacio Álvarez is currently associate professor of Food Technology and head of the Pilot Plant of Food Science and Technology at University of Zaragoza. He has been a visiting researcher at TU Berlin and Eastern Regional Research Center of the USDA. His research interest includes microbial modeling and thermal and non-thermal methods of food preservation, and particularly the inactivation of food poisoning and spoilage microorganisms by heat, ultrasound, pulsed electric fields, ionising radiation and UV-C radiation. email: [email protected]



