Sterilisation – only better
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 3 May 2005 | Wouter De Heij and Robert Van den Berg, Agrotechnology and Food Innovations b.v., Ludo Van Schepdael, Solico b.v., Hans Hoogland, Unilever Research Vlaardingen and Harmannus Bijmolt, Resato International b.v. | No comments yet
Increasing microbial safety and extending the shelf-life of packed food and other products sensitive to microbial spoilage is often performed by relatively slow thermal processes. The adverse effects of the corresponding heating periods can be decreased by using technologies that put a vast amount of energy into the product quickly, for example direct steam injection or microwave heating. Cooling the product quickly, however, is more complex.
Increasing microbial safety and extending the shelf-life of packed food and other products sensitive to microbial spoilage is often performed by relatively slow thermal processes. The adverse effects of the corresponding heating periods can be decreased by using technologies that put a vast amount of energy into the product quickly, for example direct steam injection or microwave heating. Cooling the product quickly, however, is more complex.
Increasing microbial safety and extending the shelf-life of packed food and other products sensitive to microbial spoilage is often performed by relatively slow thermal processes. The adverse effects of the corresponding heating periods can be decreased by using technologies that put a vast amount of energy into the product quickly, for example direct steam injection or microwave heating. Cooling the product quickly, however, is more complex.
Fluid foodstuffs can be flash-cooled, but this is not an option when the product is not pumpable (e.g. ready-to-eat meals and soups containing particles). The combination of high temperatures and long residence times results in a decrease in food quality. Flavours reduce, textures change, all of which creates an undesirable mouth-feel (often mushy and soft) and the change in colour can indicate a stale appearance.
In recent decades, much effort has been put into the research and development of mild pasteurisation processes. Examples of these novel technologies are Pulsed Electric Field (PEF) and High Pressure Processing (HPP) – both mild processes with known pasteurisation effects.
The development of HPP for sterilisation purposes has been focused on the combined effect of temperature and pressure. Several researchers demonstrated that HPP-sterilisation is feasible by combining both heat and pressure. Multiple consecutive pressure pulses, or long pressure holding times (>> 15 min), have proven to result in a ‘commercially-sterile’ product, but have not yet resulted in an innovative breakthrough since processing conditions are too extreme to apply them cost effectively.
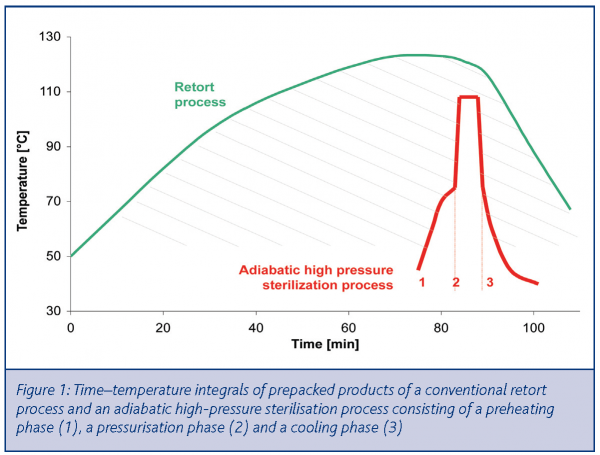
A cost-effective HPP sterilisation process may, however, be achieved according to Van Schepdael, De Heij and Hoogland (WO 02/45528)1. This patent describes an adiabatic-isobaric sterilisation process consisting of a pressure pulse (6000 – 9000 bar) with an initial-product-temperature between 60-90°C and typical treatment times between one and five minutes. Due to the optimal use of adiabatic compression, the corresponding product-temperature ranges from 100-115°C during the very short pressure pulse. As a consequence, product degradation is minimised since the time-temperature integral is very small compared to a conventional retort process (Figure 1).
Since 2000, a research consortium consisting of Unilever Research Vlaardingen, Stork Food & Dairy Systems, Agrotechnology and Food Innovations (previously known as ATO b.v.) and Resato International has been studying several aspects of high-pressure sterilisation processing, such as design and scaling of equipment; the effects on microorganisms and spores; product quality; packaging and consumer acceptance. The objective of the consortium is to develop a food-safe, cost-effective high-pressure sterilisation process that renders high quality products. This article presents an overview and describes the progress of the consortium in the field of equipment design.
Food safety aspects of HP-sterilisation
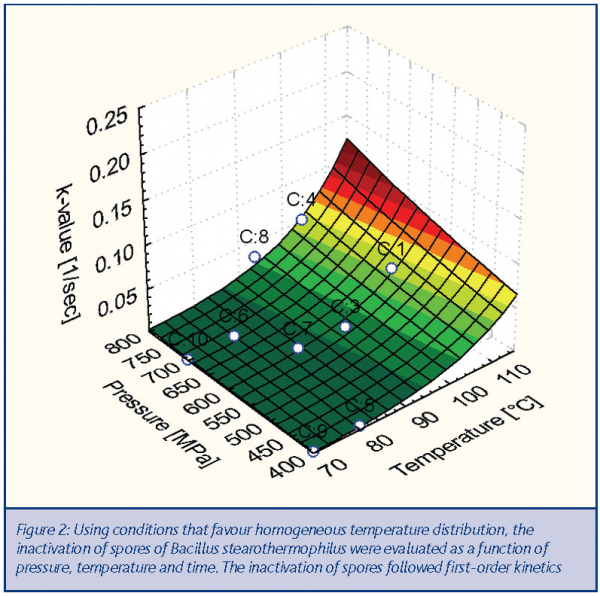
The kinetics of inactivation of Bacillus stearothermophilus spores were determined using the high pressure sterilisation labscale unit located at Agrotechnology and Food Innovations. From the observed log-reductions in viable count, the reaction rate k was plotted as a function of pressure and the product temperatures during the pressure cycle and fitted with a modified Eyring-Arrhenius equation. The 3D graph on this page demonstrates this dependency6. Based on this graph, it can be concluded that in the range of 300 – 800 MPa, the degree of spore inactivation is mainly dependent on the final product temperature and, to a lesser extent, on pressure.
It has been suggested that high-pressure sterilisation requires at least two pressure pulses because a single pressure pulse would only injure cells sub-lethally. This supposition was based on the observation that no surviving spores were present immediately after treatment, but after one week of storage, growth had occurred in similarly treated samples. The authors evaluated this phenomenon using spores of Bacillus subtilis. Serially 10-fold diluted spore suspensions of B. subtilis were prepared in growth and transferred to polyethylene pouches. A portion of the pouches were used immediately after treatment to determine the viable count by plate counting; remaining pouches were not opened but stored at 37°C. After a 60 day period of storage, the pouches were visually evaluated for growth. From the pattern of occurrence and absence of growth in the pouches, the number of surviving spores was estimated by the most probable number method (MPN) – a statistical approach for the enumeration of microorganisms in serial dilutions.
Besides B. stearothermophilus and B. subtilis spores, the consortium investigated the inactivation behavior of Cl. sporogenes and C. botulinum spores as well. Experiments conducted at both adiabatic-isobaric and isothermal-isobaric conditions have been used to determine the inactivation kinetics. The combination of plate counting and the most probable number method (MPN) under controlled HP-sterilisation conditions coupled with predictive models has resulted in a good insight in the interaction between the technology and the inactivation kinetics of bacterial spores. The detailed results of these surveys will be published soon. The predictive model may be accessed at www.virtualfactory.net
Pilot facility under construction
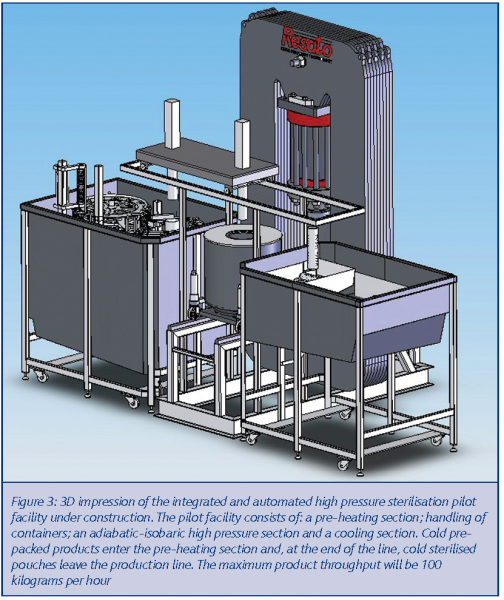
Demonstrating the potential benefits on laboratory scale is important in order to validate technological assumptions. To demonstrate the industrial benefits, however, access is required to a fully operating and integrated pilot as well. In 2003 the consortium began designing such an integrated fully-automated pilot facility, with an intended maximum product capacity of 100 kg per hour, based on the following sections:
- A continuous pre-heating (‘sous-vide’) section: in this section pouches are quickly heated to the required initial product temperature and pre-heated containers are filled with these pouches
- An automated handling system: containers filled with the pre-heated packed pouches are automatically transported from the pre-heating section to the high pressure system. After the pressure treatment the containers are transported to a cooling-section
- Batch-wise operated high pressure sterilisation machinery that is optimised thermally to operate under adiabatic-isobaric conditions
- A control and monitoring system to ensure the reproducibility of the pilot line and integration of all consecutive sections
As a starting point, the consortium pre-designed a fully functional high pressure sterilisation food factory with a capacity of 2000-4000 kg per hour. This pre-design has been downscaled to the integrated pilot facility currently undergoing construction. The system has been designed according to EHEDG hygienic design guidelines.
The main objective of this process line is to demonstrate the commercial and practical application of High Pressure sterilisation for food processing purposes. Beginning in the summer of 2005 the pilot facility will be used for the following purposes:
- To illustrate that required food-safety objectives can be realised
- To treat food products necessary to conduct consumer tests to optimise product recipes
Critical design considerations
To ensure that all product pouches have been sterilised, certain variables must be measured and recorded – much like in conventional retorting. Important critical design points for high pressure sterilisation are:
- The initial product temperature and uniformity throughout the product before entering the pressure system. The product must be at the initial target temperature and there must be no cold spots
- Temperature in the high-pressure vessel chamber prior to processing. This will ensure that the initial vessel temperature is at the proper target temperature prior to introducing the prepackaged foods
- The temperature of the pressurisation fluid and the method to pressurise the system (internal plunger system versus external high pressure pump)
- The technical solutions implemented into the pressure system to maximise the benefits of adiabatic heating during the high pressure treatment. Special care must be emphasised to heat sinks (e.g. the bottom and lid of the vessel)
Since temperature is such an import factor, the consortium explicitly decided to use the following temperature criterion: “The product temperature difference between the hottest and the coldest spot inside a pouch, during the entire process from-pre-heating-to-handling-to-pressure-treatment, may not exceed 2°C”.
The following technical solutions during pre-heating (‘sous-vide’) and handling are implemented:
- The maximum thickness of each individual pouch is controlled mechanically. The pre-heating time and temperature of the pre-heating medium are also controlled using an automated control system
- Handling time ‘from pre-heating to pressure-vessel‘ is minimised (less than 20 seconds) and during transport heat losses are avoided by means of a special constructed handling device
The following technical solutions are implemented to maximise the benefits of adiabatic heating during the pressure treatment:
- A high pressurisation rate. The pilot system has been constructed to pressurise products from 0 – 8000 bar within 30 seconds. Fast pressurisation minimises cooling during pressurisation
- Application of a liner and container materials with low heat transfer properties and with an adiabatic temperature rise similar to the food product. In general, metals may not be applied
- An optimised liner and container thickness, compared with the proper construction materials will minimise, or even prevent, cooling of the product for periods of five minutes
- Avoiding heat loss from the product to the colder pressure fluid entering the pressure vessel. Using an internal intensifier system (a plunger is used to compress the volume within the high pressure vessel) no cold pressure fluid enters the pressure vessel
- Special design effort was made to prevent axial heat losses. Both the lid and bottom of the high pressure vessel are connected with a yoke to resist the internal pressure. This construction results in a massive heat-sink. In the pilot facility a special technical solution is implemented to prevent this
Conclusions
- Temperature is the dominant factor in high-pressure inactivation of bacterial spores. The effect due to pressure may be described as a reduction of the maximum product sterilisation temperature, typically 5-15°C. Control of the temperature level and distribution of the product in the vessel, before and during pressure treatment, is essential for adequate processing3,4
- Maximising the benefits of adiabatic heating of the product allows high pressure sterilisation under milder conditions (lower pressure, treatment time and initial pressure) than described so far2
- Compared to a conventional retort process, high-pressure sterilisation involves a smaller time-temperature integral. The resulting product quality is usually better than retort treated products with less thermal degradation5
- A predictive model, in combination with a database of kinetic inactivation parameters, has proven to be a useful tool for designing the high-pressure sterilisation processes
- Validation of the HP-sterilisation process is entering the last research stage. Large datasets of microbial inactivation kinetics have already been produced and in the near future further datasets will also be published (e.g. inactivation of Clostridium botulinum spores). Validation is being carried out in multiple systems using both plate count and the Most Probable Number method
- A pilot scale continuously operated HP Sterilisation processing line, based on the currently running laboratory equipment combined with pre-treatment equipment and handling system, is under construction. All relevant HACCP and hygienic design standards and guidelines have been applied to meet the highest hygiene requirements of the food industry
This study is financially supported by the Dutch Dept. of Economic Affairs, program EETA97033. The authors thank Theo Post (Resato International b.v.), Roy Moezelaar, Remco Hamoen (Agrotechnology and Food Innovations), Huub Lelieveld and Joop Mostert (Unilever Research Vlaardingen) for their input and stimulating discussions.



References
- Van Schepdael, L.J.M.M., De Heij, W.B.C., and Hoogland, H. 2002. Method for high-pressure preservation. PCT patent application WO 02/45528 A1
- De Heij W.B.C., Van Schepdael L.J.M.M., Moezelaar R., Van den Berg R.W., Sterilization by High Hydrostatic Pressure: Increasing Efficiency and Product Quality by Improved Temperature Control, Advances in High Pressure and Bioscience and Biotechnology II (proceedings of the 2th International Conference on High Pressure BioScience and Biotechnology), 2002, R. Winter
- Sizer, C.E., Balasubramaniam, V.M., and Ting, E. 2002. Validating high-pressure processes. Food Technol. 56(2): 36-42
- Ting, E., Balasubramaniam, V.M., and Raghubeer, E. 2002. Determining thermal effects in high-pressure processing. Food Technol. 56(2): 31-35
- Matser, A.M., Krebbers, B., Van den Berg, R.W., Bartels, P.V. (2004) Advantages of high pressure sterilisation on quality of food products. Trends in Food Science & Technology 15 (2004) 79-85
- De Heij W.B.C., Van Schepdael L.J.M.M., Moezelaar R., Hoogland H, Matser A.M., van den Berg RW. 2003. High-pressure sterilization: maximizing the benefits of adiabatic heating. Food Technol. 57(3): 37–41




