Thermal processing in the food industry
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 30 April 2012 | Matteo Campagnoli, Research Manager, Barilla G&R Fratelli | No comments yet
Nowadays in the food industry, there are innovative technologies with some very interesting applications on an industrial scale and finished products on the market. In spite of this, heat remains the main process used to preserve foods. The aim of this article is to give an overview of the main thermal processes, how they relate to food safety and also to consider the management and the validation of a thermal process.
The main food safety concern related to ambient stable heat treated foods is Clostridium botulinum. Table 1 gives the most reported recent cases of product recalls due to potential contamination from C. botulinum and outbreaks caused by this microorganism. This microorganism is a spore former, highly heat resistant, grows at pH equal or higher than 4.5 and is strictly anaerobic. Therefore, if these microorganisms survive in retorted foods, and the conditions are favourable for growth, they could potentially grow in areas with an absence of oxygen. Once C. botulinum spores germinate, if they are able to generate vegetative cells and these are able to grow, they can produce lethal neurotoxins.
Due to this potential impact on food safety, C. botulinum was studied and a tailored thermal process was designed known as the ‘Botulinum Cook’. The ‘Botulinum Cook’ equals 121.1°C for three minutes, or an equivalent process.
Nowadays in the food industry, there are innovative technologies with some very interesting applications on an industrial scale and finished products on the market. In spite of this, heat remains the main process used to preserve foods. The aim of this article is to give an overview of the main thermal processes, how they relate to food safety and also to consider the management and the validation of a thermal process. The main food safety concern related to ambient stable heat treated foods is Clostridium botulinum. Table 1 gives the most reported recent cases of product recalls due to potential contamination from C. botulinum and outbreaks caused by this microorganism. This microorganism is a spore former, highly heat resistant, grows at pH equal or higher than 4.5 and is strictly anaerobic. Therefore, if these microorganisms survive in retorted foods, and the conditions are favourable for growth, they could potentially grow in areas with an absence of oxygen. Once C. botulinum spores germinate, if they are able to generate vegetative cells and these are able to grow, they can produce lethal neurotoxins. Due to this potential impact on food safety, C. botulinum was studied and a tailored thermal process was designed known as the ‘Botulinum Cook’. The ‘Botulinum Cook’ equals 121.1°C for three minutes, or an equivalent process.
Nowadays in the food industry, there are innovative technologies with some very interesting applications on an industrial scale and finished products on the market. In spite of this, heat remains the main process used to preserve foods. The aim of this article is to give an overview of the main thermal processes, how they relate to food safety and also to consider the management and the validation of a thermal process.
The main food safety concern related to ambient stable heat treated foods is Clostridium botulinum. Table 1 gives the most reported recent cases of product recalls due to potential contamination from C. botulinum and outbreaks caused by this microorganism. This microorganism is a spore former, highly heat resistant, grows at pH equal or higher than 4.5 and is strictly anaerobic. Therefore, if these microorganisms survive in retorted foods, and the conditions are favourable for growth, they could potentially grow in areas with an absence of oxygen. Once C. botulinum spores germinate, if they are able to generate vegetative cells and these are able to grow, they can produce lethal neurotoxins.
Due to this potential impact on food safety, C. botulinum was studied and a tailored thermal process was designed known as the ‘Botulinum Cook’. The ‘Botulinum Cook’ equals 121.1°C for three minutes, or an equivalent process.
In thermal processing, this ‘Botulinum Cook’ is known as F0=3 and is the minimum thermal treatment to apply in order to reach commercial sterility. It is important to underline that this processing parameter refers to moist heating conditions where the lethality follows an approximately linear correlation with time. It is also relevant to highlight that for discontinuous processes, the minimum ‘Botulinum Cook’ must be reached at the coldest point in the retort and the coldest area in the pack. Both these two cold spots must be identified with temperature profiling and the thermal process properly designed around them.
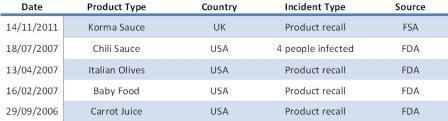

Table 1: Most recent reported cases of product recall and outbreaks from C.botulinum
Risk assessment and process design
Considering the thermal processes on a wider view, how to distinguish between pasteurisation and sterilisation? Which combinations of temperature and time define a pasteurisation rather than a sterilisation process?
Pasteurisation (processes lower than F0= 3) aims to extend the product shelf life but not to give commercial sterility, and is usually associated with other hurdles such as acidity, water activity (Aw) and refrigeration.
The design of a thermal process must start by considering the product characteristics (including raw materials and semi finished products) and then outlining what the target microorganisms are and the level of risk associated with them. There is a food safety risk if the food contains pathogens and the heat process applied allows survival or the food presents a good substrate for their growth. In addition, there can be spoilage with loss of quality if spoilage organisms can grow. Food safety always comes first and must be guaranteed at all times when designing a thermal process, however, control of spoilage during the products shelf life is of huge economic importance to the food industry. Food safety and quality can be managed not only with the thermal process but also with other hurdles such as pH, Aw and refrigeration to mention the most common.
Chilled products
For products stored chilled, with a pH greater than 4.5, an Aw higher than 0.97 and a shelf life longer than 10 days, the minimum pasteurisation required is to process at 90°C for 10 minutes or an equivalent process. The target is the elimination of spores of psychotropic C. botulinum that can grow at refrigeration temperature. Applying this process will not eliminate spores of mesophilic bacterials (for example Bacillus spp.) that can include both pathogens and spoilage organisms but the product is kept safe and stable by maintaining the chill temperature. For products following the aforesaid characteristics, but having a shelf life shorter than 10 days, it is possible to apply a milder thermal process of 70°C for two minutes that targets vegetative pathogens able to grow even at refrigeration temperatures.
Shelf stable products
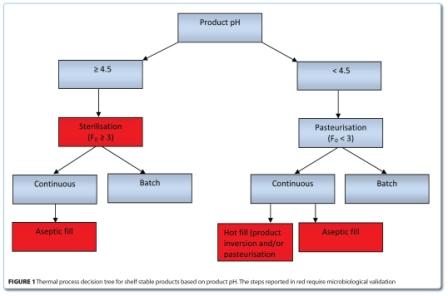

Into this category are listed both products that are pasteurised and commercially sterilised. Figure 1 shows a decision tree referring to these two processes and with concern to process microbiological validations. The main disting – uishing criterion is the pH, the important level being pH 4.5. Therefore, we have thermal processes at a minimum of F0=3 or equivalent for sterilisation and processes lower than F0=3 for pasteurisation. There are however exceptions.
When the Aw is the main factor of stability in pasteurised products, in the same way as pH, it is important to verify the value does not increase to levels permitting growth of the target microorganisms throughout the whole product shelf-life.
There is very limited literature available that relates time – temperature combinations for processing and Aw levels; this is why the primary way to determine a thermal process comes from the pH value. In these situations with limited reference, it is very important to assess the behaviour of a target microorganism in the specific product. This can be done initially in the lab at a research stage and it is very helpful for designing the appropriate thermal process. Later on, a microbial validation performed directly on pilot plants and industrial plants is the only way to verify the desired lethality is delivered.
In this case using the same approach, it is mandatory to determine the microbial behaviour throughout the whole shelf-life by doing challenge test studies.
Considering high acid products (pH < 4.5), e.g. fruit juices and soft drinks, these can all be pasteurised and stored at room temperature since the pH is the main factor for stability in addition to thermal processing. Even for a pH lower than 4.5 there are different ranges of pH values with different target microorganisms and therefore different thermal processes required. It is interesting to note that for these products, the risk involved is from spoilage only (for example acid tolerant Bacillus spp., Clostridium butyricum, yeasts and moulds).
Validation criteria and process management
Why is validating a thermal process so important? The aim of a thermal process is to increase the level of safety and quality of the finished product; therefore, it is very important to verify that the designed microbial lethality is properly delivered. From concept to product launch, there are a few main occasions when to carry out a microbiological validation. The first one is at the pilot plant stage. This is the first time when developing a product where the final recipe is put through a small scale production. It is important at this stage to verify that by setting the designed time and temperature combinations, the microbial lethality achieved inside the product is the one desired. The heat resistance of microorganisms is largely affected by the product matrix and it can be estimated at a research stage in the lab, and then used to design the minimum process in a production plant.
The actual process applied to the product in the production plant is affected by many variables involved in the design of the plant e.g. cold points, fluid flow, design and layout of heat exchangers, the diameter and length of holding tubes and so on. Microbial validations are done on continuous processing by inoculating a certain amount of product, run it through the heat exchanger and assessing the amount of microorganisms survived. Different micro – biological techniques are available to do this. For retort processing, it is generally done by inoculating several pouches or cans and analysing them for survivors at the end of the process. Another reason for validating a thermal process at this stage is that productions from pilot plants may be used for sensory tests and therefore safety is of utmost importance. Since pilot plants are usually part of the Research & Development Area facilities, it is relatively easy to carry out microbiological validations. The scenario may be more complicated when validating a plant on an industrial scale.
For thermal processes that have food safety as a target, it is also very important to carry out microbial validations in industrial plants. It should be noted that a successful validation on a pilot plant scale does not automatically have as a direct result success at the industrial scale, even if the only difference between the two plants is the size, there is no guarantee to achieve the same level of microbial lethality and so it should be confirmed by actually measuring the lethality. For pasteurisation of high acid products (pH < 4.5) that have not got food safety as the target, it is sufficient to validate them by verifying that the correct temperature and time combinations are applied. For these processes, it is important to assess the lethality achieved at the head space area for hot filling during inversion and / or post filling pasteurisation, as the filling is a critical point of the line where product contamination can happen. If there is aseptic filling, a microbiological validation of the filling area sterilisation procedure as well as the packaging must be carried out to assess the lethality achieved by using chemicals at a specific concentration. In cases where such validations are not possible, a way to assess the efficacy of the thermal process is to carry out sterility or commercial stability tests on finished products. These indirect validations are very demanding in terms of number of samples and therefore expensive. Furthermore, there is not a direct test of the thermal process so an area of uncertainty is always present.
Conclusion
A deep and thorough understanding of process performance and management of critical points is mandatory to achieve good level of safety and this can be extended to every process that is crucial in terms of food safety. Judgments on the level of food safety achieved on finished products must be based on processes that are microbiologically validated and are not only based on sampling plans and testing. Relying on testing only is a very risky practice since it does not provide information about the lethality of the process in place and it is also affected by the probability and uncertainty that every sampling plan takes in.
Another very important reason for doing microbiological validations of industrial processes is the possibility to define exactly the minimum acceptable processing conditions that deliver a specific level of microbial kill and be able to scientifically assess potential process deviations and make a clear judgement on finished product acceptance.
References
- Da-Wen Sun, (2009). Engineering Aspects of Thermal Food Processing. CRC Press, Ricardo Simpson
- J.E. Gaze, (2006). Pasteurisation: A Food Industry Practical Guide. Second edition, Campden & Chorleywood Food Research Association Group
- CDC (Centre for Disease Control and Prevention) (2007). Botulism Associated with Canned Chili Sauce, July-August 2007. Available at: http://www.cdc.gov/botulism/botulism.htm. Accessed December 28, 2011
- PritzkerOlsen, P.A. Botulism Outbreaks and Recalls. Available at: http://www.pritzkerlaw.com/botulismoutbreaks/ . Accessed December 28, 2011
About the author
Since June 2011, Matteo Campagnoli has covered the role of Research Manager at Barilla G. & R. Fratelli within the area of thermal processing and microbiology. Before this, Matteo was a Technical Officer at Campden BRI in the Microbiology and Heat Resistance Group where he specialised in process validations and food safety for thermally processed foods.




