Profiling of intact wheat arabinoxylans by spectroscopy
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 6 September 2011 | Gwénaëlle Le Gall, Peter R. Shewry, E.N. Clare Mills and Geraldine A. Toole, Institute of Food Research | No comments yet
Wheat is the most widely grown cereal in the world and is used to make a variety of baked goods, such as bread, biscuits, pasta, noodles and breakfast cereals. On hydration of flour to make a dough, the seed storage proteins form a cohesive mass known as gluten. This protein fraction has a unique structure and viscoelastic properties1 that have allowed wheat to be used in such a versatile way. Dough properties vary between different cultivars and wheat lines – making some of them more suitable for pasta, others bread and others biscuits. Often, these products are made from white flour, and yet it is clear that there are beneficial effects of having a diet rich in whole grain and fibre derived from the endosperm cell walls and the outer layers of the grain that are found in the bran fraction. Whilst the variation in properties of gluten components has been extensively described, variation in cereal cell wall composition has been less studied yet might make an important contribution to improving the nutritional quality of cereal foods2.
Wheat is the most widely grown cereal in the world and is used to make a variety of baked goods, such as bread, biscuits, pasta, noodles and breakfast cereals. On hydration of flour to make a dough, the seed storage proteins form a cohesive mass known as gluten. This protein fraction has a unique structure and viscoelastic properties1 that have allowed wheat to be used in such a versatile way. Dough properties vary between different cultivars and wheat lines – making some of them more suitable for pasta, others bread and others biscuits. Often, these products are made from white flour, and yet it is clear that there are beneficial effects of having a diet rich in whole grain and fibre derived from the endosperm cell walls and the outer layers of the grain that are found in the bran fraction. Whilst the variation in properties of gluten components has been extensively described, variation in cereal cell wall composition has been less studied yet might make an important contribution to improving the nutritional quality of cereal foods2.
Wheat is the most widely grown cereal in the world and is used to make a variety of baked goods, such as bread, biscuits, pasta, noodles and breakfast cereals. On hydration of flour to make a dough, the seed storage proteins form a cohesive mass known as gluten. This protein fraction has a unique structure and viscoelastic properties1 that have allowed wheat to be used in such a versatile way. Dough properties vary between different cultivars and wheat lines – making some of them more suitable for pasta, others bread and others biscuits. Often, these products are made from white flour, and yet it is clear that there are beneficial effects of having a diet rich in whole grain and fibre derived from the endosperm cell walls and the outer layers of the grain that are found in the bran fraction. Whilst the variation in properties of gluten components has been extensively described, variation in cereal cell wall composition has been less studied yet might make an important contribution to improving the nutritional quality of cereal foods2.
In order to address this gap, researchers at the Institute of Food Research (IFR) have been applying spectroscopic techniques developed to study intact plant cell walls, such as Fourier Transform Infra Red (FT-IR) and FT-Raman over a decade ago3 to gain insight into the structure of wheat endosperm cell walls4. Recently, this has been extended to include high resolution 1H Nuclear Magnetic Resonance (1H NMR) spectroscopy. These sophisticated tools have been used to study how cell wall composition alters during grain development and to define the structural variations found between cell wall polymers in different wheat varieties or cereal species, such as barley, rye and oats. This paper summarises recent studies conducted by IFR scientists and colleagues on the structural analysis of cell walls from grains of wheat and other cereals and highlights the promising potential of spectroscopic techniques to address problems associated with human health, food security and sustainability.
Composition of endosperm cell walls
Around 12 per cent of the mature wheat grain is composed of cell wall polysaccharides which consist mainly of arabinoxylans (AX)5. Structurally, these polymers are made up of a backbone of (1→ 4) linked β-D-xylopyranosyl residues either unsubstituted, or monosubstituted at the O-3 position or disubstituted at the O-2 and O-3 positions with α-L-arabinofuranosyl units. Cereal AX exhibit a high degree of endogenous microheterogeneity6 both spatially and compositionally and this characteristic has significant impact on the utilisation of the grain. The high water holding capacity of both soluble and insoluble AX directly affects the quality of food products via its effect on dough properties7. Studies on the functional effects of AX have been carried out since the 1970s8,9, and the detailed analysis of their composition has been achieved through enzyme digestion which resulted in oligomers which were fractionated by preparative liquid chromato – graphy and characterised by 1H NMR10,11. Extensive use of this procedure led Gruppen et al12 to propose a model for wheat AX consisting mainly of repetitive patterns of highly-branched regions enriched in both O-2,3 disubstituted, as well as O-3 monosubstituted xylose residues and less-branched regions.
Spectroscopies to study arabinoxylans (AX) in intact cell wall extracts
In the mid 1980s, a research group at IFR pioneered the use of FT-IR and Raman spectro – scopies to study extracted polysaccharides13. The methods were particularly suited to detect and analyse changes to the three-dimensional arrangement of polysaccharide chains that accompany gel formation or to quantify the relative proportions of chains in crystalline and amorphous regions of a polymer like starch. This versatility led to numerous FT-IR and Raman-based IFR research studies covering food authentication14, food structure15 and plant cell wall architecture16.
Profiling of complex extracts by 1H NMR was developed in parallel with FT-IR. In the early 1990s, a group from London led by Professor Jeremy Nicholson forged a new way of profiling mammalian biofluids using 1H NMR and applied multivariate analysis to highlight any similarities or differences between sample groups (e.g. healthy versus diseased)17. The concept was rapidly applied to food material at IFR and Belton et al.18 first used it to probe varietal differences between apples in 1998. The NMR group developed over the years an expertise in profiling all types of food (fruits19, vegetables, cheese, meat20, wine) and plant21 material to tackle authentication and substantial equivalence issues. With the acquisition of a 600 MHz NMR spectrometer in 2005, the research themes expanded to biological extracts including samples from human, animal and in vitro studies of bacteria and mammalian cells.
Applications to studying wheat endosperm cell walls during grain development
In 2007, using an FT-IR spectrometer equipped with a microscope it was shown that the AX in the endosperm cell-wall of wheat exhibit a high degree of spatial heterogeneity, and that the structures of the AX polymers change during grain development from a highly substituted to a lower substituted form22 as denoted by changes in the FT-IR spectrum. The rate of change was found to be faster in the plants grown at higher temperature with limited water supply from 14 days post anthesis (dpa) (Figure 2). The grain development under cold/wet versus hot/dry conditions was also studied using Raman microspectroscopy6 revealing slight differences in the arabinose:xylose (A/X) ratio between the two growth conditions and a lower esterification with ferulic acid for the hot/dry conditions. This observation, supported by earlier work, suggested that AX is either delivered to the cell wall highly substituted and is then remodelled through the action of various enzymes, or brought at a low level of substitution into the wall at a later stage of development. 1H NMR applied to the same samples confirmed that the A/X ratio did not change greatly. However, because of its superior resolution compared to FT-IR and Raman spectroscopy, it showed that the ratio of mono- to di-substituted Xylp residues increased by fourfold at maturity, explaining the decrease in highly branched AX (HB-AX) over time observed by FT-IR (Figure 2).
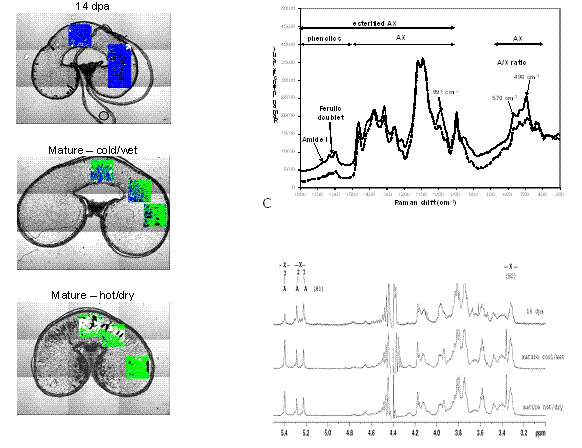

Figure 2 A. FT-IR spectroscopic images superimposed onto visible images from transverse cell wall-only thin sections (all cell contents removed, to leave only the cell wall network, showing three cell types; subaleurone, prismatic and round cells). Regions in colour represent the areas that were spectroscopically mapped. A colour was assigned to each pixel (16,384 pixels per image); green to represent low branched AX (LB-AX); blue to represent highly branched AX (HB-AX); white represents remaining starch, and black represents holes or pixels where the amount of AX was too low to determine. B. Typical Raman spectra for the endosperm cellwall of wheat at maturity (straight line) and at 14 days post anthesis (dpa) (dashed line), C. 1H NMR spectra for endosperm cell-wall of wheat at 14 dpa and at maturity under cool/wet or hot/dry conditions (200 transverse cell wall-only thin sections for each stage of grain development were allowed to dry and dissolved in D2O)
FT-IR and 1H NMR spectroscopy were also applied to a UK standard breadmaking wheat variety (cv. Hereward) to study the grain filling period in detail5. Similar temporal changes to the ones observed previously were observed for this variety, namely a reduction in disubstitution and an increase in monosubstituted xylose residues. The remodelling occurred gradually between 12 dpa and 17 dpa with little change between 17 dpa and maturity (~48 dpa) (Figure 3). Through collaboration with INRA, the proportions of AX and β-glucan (BG) present during the grain filling period were determined using enzymatic fingerprinting. It showed that the amount of both non-starch polysaccharides increased over time and that the AX: β-glucan ratio increased from 1:1 at 12 dpa to 3:1 at 35 dpa with no further changes subsequent to that5. Enzymatic fingerprinting confirmed the differences in cell wall composition mapped by FT-IR between the early stages of development (10 and 12 dpa) and the later stages (17 – 42 dpa). This was due to a higher proportion of BG and highly substituted oligomers at 10 and 12 dpa, and a lower proportion of β- glucan (G3+G4) and AX with less arabinose substitution5 later on, information which is further relayed by the 1H NMR results (Figure 3).
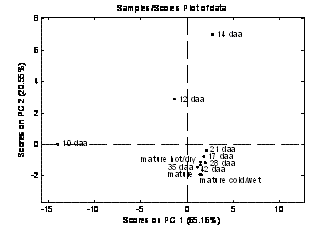

Figure 3 Principal Component Analysis (PCA) for the 1H NMR spectra of bread wheat cv. Hereward cell wall extracts at several developmental stages (10, 12, 14, 17, 21, 28, 35, 42 and 48 dpa (maturity)). The PCA plot shows a progressive change in the AX structure with time (an increase of monosubstituted xylose residues and a decrease of disubstituted xylose residues) reaching an AX profile at 17 dpa that remains stable until maturity.
Variation in cell wall composition across wheat varieties and cereal species
More recently, we conducted a survey of the composition of the endosperm cell wall of many wheat varieties in a large diversity collection, which was studied as part of the EU 6th Framework project ‘HEALTHGRAIN’23,24. Fifty bread wheat lines were screened using FT-IR spectroscopic mapping and enzymatic fingerprinting. The approaches gave similar results, with the majority of the cultivars falling in the central part of the PCA plot24, while a minority of wheats were located away from the centre highlighting differences in AX composition (data not shown). On the basis of this screen four modern breadmaking wheat lines from France (Soissons), UK (Hereward), Italy (Manital) and China (Yumai34) and two other lines (a transitional Italian bread wheat variety San Pastore and a UK modern biscuit wheat Claire) were chosen for further detailed analysis. FT-IR and 1H NMR analysis both suggested that the cell walls of the two Italian varieties contained a high amount of HB-AX while Claire and Yumai34 consisted almost entirely of LB-AX25.
Also within HEALTHGRAIN, a comparative study was implemented on different cereals23 profiling of bread wheat lines (including spelt) alongside rye, barley, oats and various other types of ancient wheat species (monococcum, diccocum ). This was done to evaluate the degree of variability in the amount of AX and β-glucan and also the type of AX present in the cell walls. We showed that, as expected, oat cell walls are mainly composed of BG whilst the cell walls of rye are similar to wheat with a high proportion of AX26. To illustrate these results, PCA was applied to the 1H NMR spectra from all cereals studied plus wheat (cv. Hereward) at different stages of development (Figure 4). It is interesting to note that the scores of all the wheat samples cluster close together on the PCA plot regardless of the variety and the environmental conditions and that the score for the immature wheat (10 dpa) stands alone away from the cluster of scores representing the wheat at >10 dpa (Figure 4), demonstrating how structurally different the immature grain is compared to grains at 12 dpa and after.
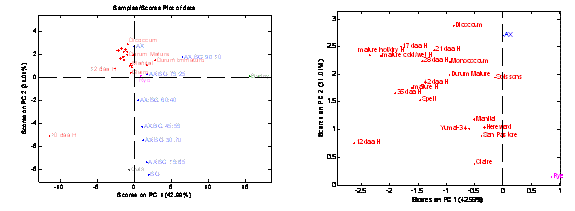

Figure 4 PCA for the 1H NMR spectra of cell wall extracts for several varieties of wheat, cv. Hereward (H) at various stages of development and environmental conditions. Spectra of other cereals such as rye, barley and oats and a series of AX and β-glucan (BG) standards which vary in proportions of the two non-starch polysaccharides are also included to highlight the compositional differences between the various cereals. Wheat at 10 dpa differs remarkably from other stages of development due to a different AX structure
New development: studying arabinoxylans extracted from dough
A new, high-throughput method has been developed whereby intact endosperm cell walls may be extracted from a wheat flour dough. The extracted endosperm cell-wall ’gel’ was analysed using FT-IR spectroscopic imaging and multivariate statistics. Differences in arabinoxylan (AX) structure and composition were determined for flours produced from grains grown under differing environmental conditions. Cultivars with known differences in cell wall AX composition were also analysed and the results compared with those achieved during earlier studies6,22. This appears to be an extremely effective way to extract large amounts of endosperm cell walls from wheat flour with minimum interference or use of chemical procedures. The extracted endosperm cell-wall ’gel’ has also been subjected to in vitro simulated biochemical gastro-duodenal digestion. FT-IR spectroscopic imaging was used to examine changes in AX structure and composition at various stages during the digestion process. Light and Atomic Force Microscopy (AFM) have been used in order to visualise these changes, and these initial digestion studies have provided extremely promising results.
Future
Cell-wall AX is an important part of the wheat grain that warrants further studies. Besides the fact that the composition and the proportion of AX can affect the quality of this worldwide staple food, AX are also a significant source of dietary fibre for humans. Animal studies have started to delineate how the structural characteristics of different oligosaccharides (breakdown products of AX) affect prebiotic properties and fermentation products in the gut27. It is estimated that the world population could reach up to nine billion by 2050 and increased yield of good quality wheat varieties could be a step towards tackling food security, although such an achievement is currently challenging, both in terms of technology and sustainability28. The spectroscopic tools described here and novel methods for isolating the cell walls from wheat doughs will facilitate future studies now underway on the effects of digestion on cell wall structure being undertaken through a BBSRC-funded project. This will allow us to investigate how the gastrointestinal environment (pH, biosurfactants, and various hydrolases) may affect the AX components of the endosperm cell walls.
Such studies are complementary to those being undertaken to develop ways of utilising sugars from edible plant crops for biofuel by increasing research to produce ethanol from lignocellulosic materials of non-food crops and the waste material of edible crops2,29,30. More research on the degradation of cell wall material such as lignins and AX is needed to address the huge challenge of producing green energies in an economically sustainable manner. Understanding the biosynthesis of cell-wall polysaccharides in the developing wheat grain, and how this is affected by developmental, genetic and environmental factors, is therefore crucial if we are to develop novel wheat cultivars and products that tackle food security, human health and large scale energy production. Acknowledgement The authors wish to acknowledge colleagues from the EU FP7 project called HEALTHGRAIN for growing and supplying wheat samples. The BBSRC DRINC project on improvement of the nutritional quality of bread is BB/I0061/09/1.
References
1. Hoseney, R.C. & Rogers, D.E. (1990) The formation and properties of wheat flour doughs. Crit. Rev. Food Sc.i Nutr. 29, 73-93
2. Xie, G. & Peng, L. (2011) Genetic engineering of energy crops: a strategy for biofuel production in China.J. Integr. Plant Biol. 53, 143-150
3. Kacurakova, M., Wellner, N., Ebringerova, A., Hromadkova, Z., Wilson, R.H. & Belton, P.S. (1999) Characterisation of xylan-type polysaccharides and associated cell wall components by FT-IR and FTRaman spectroscopies. Food Hydrocolloid 13, 35–41
4. Barron, C., Parker, M.L., Mills, E.N.C., Rouau, X. & Wilson, R.H. (2005) FT-IR imaging of wheat endosperm cell walls in situ reveals compositional and architectural heterogeneity related to grain hardness. Planta 220, 667–677
5. Toole, G.A., Le Gall, G., Colquhoun, I.J., Nemeth, C., Saulnier, L., Lovegrove, A., Pellny, T., Freeman, J., Mitchell, R.A.C., Mills, E.N.C. & Shewry, P.R., (2010) Compositional and spatial changes in cell wall composition in developing grains of wheat cv. Hereward. Planta 232, 677-689
6. Toole, G.A., Barron, C., Le Gall, G., Colquhoun, I.J., Shewry, P.R. & Mills, E.N.C. (2009) Remodelling of arabinoxylan in wheat (Triticum aestivum) endosperm cell walls during grain filling. Planta 229, 667–680
7. Simsek, S., Whitney, K.L., Ohm, J.B., Anderson, J. & Mergoum, M. (2011) Refrigerated dough quality: effect of environment and genotypes of hard red spring wheat. J. Food Sci. 76, S101-107
8. Andrewartha, K.A., Phillips, D.R. & Stone, B.A. (1979) Solution properties of wheat-flour arabinoxylans and enzymically modified arabinoxylans. Carbohydr. Res. 77, 191–204
9. Kim, S.K. & D’Appolonia, B.L. (1977) Bread staling studies .3. Effect of pentosans on dough, bread, and bread staling rate. Cereal Chem. 54, 225–229
10. Broberg, A.,Thomsen, K.K. & Duus, J.O. (2000) Application of nano-probe NMR for structure determination of low nanomole amounts of arabinoxylan oligosaccharides fractionated by analytical HPAEC-PAD. Carbohydr. Res. 328, 375–382
11. Gruppen, H., Hoffmann, R.A., Kormelink, F.J.M., Voragen, A.G.J., Kamerling, J.P. & Vliegenthart, J.F.G. (1992) Characterisation by 1H NMR spectroscopy of enzymically derived oligosaccharides from alkaliextractable wheat-flour arabinoxylan. Carbohydr. Res. 233, 45–64
12. Gruppen, H., Kormelink, F.J.M. & Voragen, A.G.J. (1993) Water-unextractable cell wall material from wheat flour. 3. A structural model for arabinoxylans. J. Cereal Sci. 18, 111–128
13. Belton, PS, Wilson, R.H. & Chenery, D.H. (1986) Interaction of group-i cations with iota-carrageenans and kappa-carrageenans studied by fourier-transform infrared-spectroscopy. Int. J. Biol. Macromol. 8, 247-251
14. Al-Jowder, O., Defernez, M., Kemsley, E.K. & Wilson, R.H. (1999) Mid-infrared spectroscopy and chemometrics for the authentication of meat products. J. Agric. Food Chem. 47, 3210-3218
15. Salt, L.J., Wilde, P.J., Georget, D., Wellner, N., Skeggs, P.K. & Mills, E.N.C. (2006) Composition and surface properties of dough liquor J. Cereal Sci. 43, 284-292
16. Sene, C., McCann, M.C., Wilson, R.H. & Grinter R. (1994) Fourier-Transform Raman and Fourier-Transform Infrared Spectroscopy (An Investigation of Five Higher Plant Cell Walls and Their Components).Plant Physiol. 106, 1623-1631
17. Holmes, E., Foxall, P.J.D., Nicholson, J.K., Neild, G.H., Brown, S.M., Beddell, C.R., Sweatman, B.C. Rahr, E., Lindon ,J.C., Spraul, M. & Neidig,P. (1994) Automatic data reduction and pattern-recognition methods for analysis of H-1 nuclear-magnetic-resonance spectra of human urine from normal and pathological states. Anal. Biochem. 220, 284-296
18. Belton, P.S., Colquhoun, I.J., Kemsley, E.K., Delgadillo, I., Roma, P., Dennis, M.J., Sharman, M., Holmes, E., Nicholson, J.K. & Spraul, M. (1998) Application of chemometrics to the H-1 NMR spectra of apple juices: discrimination between apple varieties Food Chem. 61, 207-213
19. Le Gall, G., Colquhoun, I.J., Davis, A.L., Collins, G.J. & Verhoeyen, M.E. (2003) Metabolite profiling of tomato (Lycopersicon esculentum) using 1H NMR spectroscopy as a tool to detect potential unintended effects following a genetic modification. J. Agric. Food Chem. 51,2447-2456
20. Al-Jowder, O., Casuscelli, F., Defernez, M., Kemsley, E.K., Wilson, R.H. & Colquhoun, I.J. (2001) High resolution NMR studies of meat composition and authenticity Magn. Res. Food Sci. 262, 232-238
21. Le Gall, G., Metzdorff, S.B., Pedersen, J., Bennett, R.N. &Colquhoun, I.J.. Metabolite profiling of Arabidopsis thaliana (L.) plants transformed with an antisense chalcone synthase gene (2005) Metabolomics 1,191-198
22. Toole, G.A., Wilson, R.H., Parker, M.L., Wellner, N.K., Wheeler, T.R., Shewry, P.R. & Mills, E.N.C. (2007) The effect of environment on endosperm cell-wall development in Triticum aestivum during grain filling: an infrared spectroscopic imaging study. Planta 225:1393–1403
23. Ward, J.L., Poutanen, K., Gebruers, K., Piironen, V., Lampi, A.M., Nyström, L., Andersson, A.A., Aman, P., Boros, D., Rakszegi, M., Bedo, Z. & Shewry, P.R.(2008) The HEALTHGRAIN Cereal Diversity Screen: concept, results, and prospects. J. Agric. Food Chem. 56, 9699-709
24. http://www.healthgrain.org/
25. Toole, G.A., Le Gall, G., Colquhoun, I.J., Drea, S., Opanowicz, M., Bedő, Z., Shewry, P.R. & Mills, E.N.C. Spectroscopic analysis of diversity of arabinoxylan structures in endosperm cell 1 walls of cereal species in the HEALTHGRAIN diversity collection 2011 J. Ag. Food Chem. Accepted
26. Toole, G.A., Le Gall, G., Colquhoun, I.J., Johnson, P., Bedő, Z., Saulnier, L., Shewry, P.R. & Mills, E.N.C. Spectroscopic analysis of diversity of arabinoxylan structures in endosperm cell walls of wheat varieties (Triticum aestivum) in the HEALTHGRAIN diversity collection J. Ag. Food Chem. Accepted
27. Van Craeyveld, V., Swennen, K., Dornez, E., Van de Wiele, T., Marzorati, M., Verstraete, W., Delaedt, Y., Onagbesan, O., Decuypere, E., Buyse, J., De Ketelaere, B., Broekaert, W.F., Delcour, J.A. & Courtin, C.M. (2008) Structurally different wheat-derived arabinoxylooligosaccharides have different prebiotic and fermentation properties in rats. J. Nutr. 138,2348-55
28. Foulkes, M.J., Slafer, G.A., Davies, W.J., Berry, P.M., Sylvester-Bradley, R., Martre, P., Calderini, D.F., Griffiths & S., Reynolds, M.P. (2011) Raising yield potential of wheat. III. Optimizing partitioning to grain while maintaining lodging resistance. J. Exp. Bot. 62, 469-86
29. Hahn-Ha gerda,l B., Galbe, M., Gorwa-Grauslund, M.F., Liden, G. & Zacchi, G. (2006) Bio-ethanol—the fuel of tomorrow from the residues of today. Trends Biotechnol. 24:549–556
30. Jenkins, T., Bovi, A. & Edwards, R.. (2011) Plants: biofactories for a sustainable future? Philos. Transact. A Math. Phys. Eng. Sci. 369.1826-39
Gwénaëlle Le Gall gained a food industry sponsored PhD in chemistry from the University of East Anglia on the authentication of food using 1H NMR and multivariate statistics. Gwénaëlle has over 10 years experience in NMR and MS-based metabolomics at the Institute of Food Research (IFR) based in Norwich. She has developed expertise to cover the study of a broad range of complex extracts including food, plant, mammalian and microbial extracts email: [email protected]
Clare Mills’s research is focused around protein biochemistry and physical biochemical approaches. Clare leads the Food Structure and Health programme at IFR which seeks to gain an understanding of the rules governing the assembly of natural and fabricated food structures, their subsequent disassembly during digestion and uptake by the gut epithelium. The group is now seeking to discover why certain protein scaffolds dominate known allergens from foods. In relation to food allergy, Clare leads the EuroPrevall project worth EUR 14.25 million with 63 partners from across Europe to tackle the problems of food allergy (http://www.europrevall.org/), and has around 100 refereed publications.
Peter Shewry is a research leader at the Rothamsted Research Institute focusing on improving the end-use quality of wheat using a combination of transcriptomic and biochemical approaches and establishing novel approaches to improvement including mutagenesis, TILLING (targeted selection of mutations) and improved transformation systems. He is the author of more than 350 papers in international journals and editor or co-editor of 18 books. As well as his successful research career, Professor Shewry was for 14 years the Director of Long Ashton Research Station and more recently the Acting Director at Rothamsted Research.
Geraldine Toole completed a degree and MSc in chemistry, followed by three years working in industry, then a PhD in physical biochemistry, studying the fracture properties of plant cell walls using FT-IR. Since then she has spent the last 10 years studying the structure and composition of cereal endosperm cell walls using FT-IR techniques and is currently expanding the use of these methods to investigate how differing non-starch polysaccharides, with health beneficial properties, may be affected by processing, baking and ultimately digestion, and whether variations in cell-wall structure have any impact on their behaviour in the GI tract. email: [email protected]




