How to compare novel and conventional processing methods in new product development: A case-study on orange juice
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 4 November 2010 | Ariette Matser & Hennie Mastwijk, Wageningen UR and Diána Bánáti, Director General, Central Food Research Institute and Liesbeth Vervoort & Marc Hendrickx, Katholieke Universiteit Leuven | 1 comment
The overall objective of the EU FP6 NovelQ Integrated Project was to formulate strategic solutions for technical and basic research hurdles to enhance the development and successful demonstration of Novel Processing (NP) schemes. A parallel approach was chosen based on providing a sound scientific base and technology transfer.
The first approach has generated new insights for mechanistic and kinetic aspects on the impact of novel technologies on food safety and quality as a basis for process and product development. The second has led to integrated product and process development, and demonstration trajectories. It has also resulted in enhanced implementation of NP.
The overall objective of the EU FP6 NovelQ Integrated Project was to formulate strategic solutions for technical and basic research hurdles to enhance the development and successful demonstration of Novel Processing (NP) schemes. A parallel approach was chosen based on providing a sound scientific base and technology transfer. The first approach has generated new insights for mechanistic and kinetic aspects on the impact of novel technologies on food safety and quality as a basis for process and product development. The second has led to integrated product and process development, and demonstration trajectories. It has also resulted in enhanced implementation of NP.
The overall objective of the EU FP6 NovelQ Integrated Project was to formulate strategic solutions for technical and basic research hurdles to enhance the development and successful demonstration of Novel Processing (NP) schemes. A parallel approach was chosen based on providing a sound scientific base and technology transfer.
The first approach has generated new insights for mechanistic and kinetic aspects on the impact of novel technologies on food safety and quality as a basis for process and product development. The second has led to integrated product and process development, and demonstration trajectories. It has also resulted in enhanced implementation of NP.
Within NovelQ, evaluating the general applicability and validity of several NP schemes was defined as an important task. The effects of NP and conventional processing have been compared using well-defined processing conditions, resulting in guidelines for the application of NP schemes by food manu – facturers. These guidelines integrate the results of various activities within NovelQ involving several partner organisations. More specifically, a fruit-based product, orange juice, was selected and processed with conventional heat treatment, high pressure (HP) treatment and pulsed electric field (PEF) treatment. Process impact and homogeneity were monitored, the effects on shelf life and product quality evaluated and sensorial analysis carried out. This article describes the approach chosen and gives an overview of the results. Detailed results will be described elsewhere in scientific publications.
How to compare the effects of novel processing and conventional processing on products?
When industry is interested in evaluating the potential of NP for a specific (or new) product, the first step is often a feasibility study to obtain an impression of the effects of the specific technology on the product characteristics including shelf life and (sensorial) quality. Comparison of the effects of NP technologies with the effects of conventional preservation treatments is crucial, and in some cases, an untreated product will serve as a reference. Selection of specific process conditions used both for the novel process as well as for the conventional process is important for a correct and fair comparison. For example, comparison of a very mild novel process with a traditional / conventional process, which causes overprocessing, may result in a biased (positive) view of the novel processing. In general, state-of-the-art conventional processing has to be used for proper evaluation. However, selecting the appropriate processes (tech – nologies, sequences and conditions) is complex. The first problem is choosing an equivalent processing level for all the processing tech – nologies. Different targets can be used as a reference point including food safety (pathogens), food spoilage (spoilage micro-organisms, enzymes) and food quality.
The main criterion for choosing process conditions is food safety, according to EC regulation EC 2073/2005 Microbial criteria for Foodstuffs. A more practical recommendation is the FDA guidelines documented in Juice HACCP, which requires a five-log reduction of the pathogen identified as the ‘pertinent microorganism’, which is the most resistant microorganism of public health significance that is likely to occur in the juice. For orange juice, this is E. coli O157:H7. Therefore, the processing conditions should be the minimum required to guarantee a five-log reduction in E. coli O157:H7. Table 1 gives an overview of the processing conditions to achieve this equivalence in safety. Furthermore, the conditions should be selected in such a way that the quality aspects of the product (e.g. colour, overall liking) are as high as possible. Equivalent processing levels with respect to inactivation of enzymes or other quality affecting components can result in a different set of process conditions.
Table 1. Comparison of the processing conditions necessary to achieve a 5 log reduction of E. coli O157:H7 in orange juice and industrial conditions for chilled orange juice
| Technology | Food safety conditions | Industrial conditions |
| Fresh juice | Limited shelf life | 3-8 days shelf life |
| Heat pasteurisation | 3-6 s treatment at 71.1 °C* | 10-30 s treatment at 72-87 °C |
| High pressure pasteurisation | 1-5 min treatment at 350-500 MPa | 1 min treatment at 600 MPa |
| Pulsed electric field treatment | Unknown **) | 40 microsec at 20 kV/cm |
The second issue that has to be considered is the safety margin. In industry, a safety margin is applied to avoid the risk of insufficient processing. To determine such a margin, the coldest spot designated equipment is determined and, based on this, the processing conditions selected. However, this will result in considerable over-processing of a major proportion of food in, for example, pasteurisation of packed products. For novel processing, the safety margins are more difficult to identify and often not included in the selection of appropriate conditions. Table 1 gives examples of industrial process conditions for the selected technologies, and shows large differences amongst process conditions compared with the conditions sufficient to achieve equivalent safety levels.
The third issue is scale up of processing. Often, pilot-scale equipment is used to develop NP since industrial-scale equipment is not always available. Process conditions at pilot-scale can differ from industrial-scale equipment, for example, differences in pressure build up rate in high pressure processing, and heat transfer and packaging sizes in conventional heat processes. Attention should be paid to unrealistic comparisons or conditions that cannot be achieved at industrial-scale.
In addition to this, recipes and packaging may be different for each, for example, pre-cooking of a pasta dish may be different for heat processes compared with high pressure processing and the taste balance can be altered by specific processes resulting in the need for different amounts of seasoning (i.e. herbs, spices or salt).
NovelQ approach for comparison of effects of NP on orange juice
As described above, the aim of the integrated demonstrations within NovelQ was to evaluate the general applicability and validity of NP schemes by comparing the effects of NP and conventional processing at equivalent levels. As pulsed electric field treatment was included in the study, a liquid food product was chosen which already exists on the market, preserved using conventional technologies. In this study, orange juice was treated with high pressure, pulsed electric field treatment and heat pasteurisation, and untreated juice was used as a reference. As both HP and PEF result in a product that requires chilled storage, a heat pasteurisation process was selected on the basis of comparable food safety levels. Commercially available oranges, currently used for fresh juice, were selected for this test. Processing conditions were selected as having a similar impact with respect to food safety. Thus, in this study, orange juice processing by HP, PEF and conventional thermal processing were compared under similar conditions of low thermal impact and chilled storage conditions during shelf life. Conditions close to the food safety conditions in Table 1 were used, although these conditions may need to be adjusted scaling up to industrial levels. After processing, the samples were distributed to NovelQ partners KU Leuven (Belgium), KÉKI (Hungary), Life-UC (Denmark) and Wageningen UR (The Netherlands). Logistics were important to assure quality and safety levels were guaranteed and monitored well during transport.
As samples were to be used for sensory evaluation, equivalent processing levels were selected whilst ensuring food safety was guaranteed. The absence of pathogens was explicitly checked. Directly after processing, all samples were within required safety limits according to EC 2073/2005. During shelf life, additional microbial evaluations were carried out in triplicate for each treated orange juice with respect to aerobic mesofilic plate count, Enterobacteriaceae, yeasts and moulds, and lactic acid bacteria. As expected, the untreated juice was spoilt between 9 and 20 days during storage at 4°C. No spoilage was found after two months storage at 4°C with HP, PEF and mild heat pasteurisation.
Cloud stability, and in particular cloud separation, is an important quality aspect for orange juice and determining physical stability during shelf life. As this process is caused mainly by residual enzyme activity, enzyme inactivation during processing and temperature during shelf life are the most important factors for shelf life. Figure 1 shows pictures taken during the shelf life tests. Cloud stability was highest for heat pasteurised juice and HP, but considerably lower for PEF treated juices. The untreated juice shows the fastest cloud separation and, after nine days, microbial spoilage caused disturbance of the cloud. These results are in line with the activity of the major enzyme involved, pectinmethylesterase.
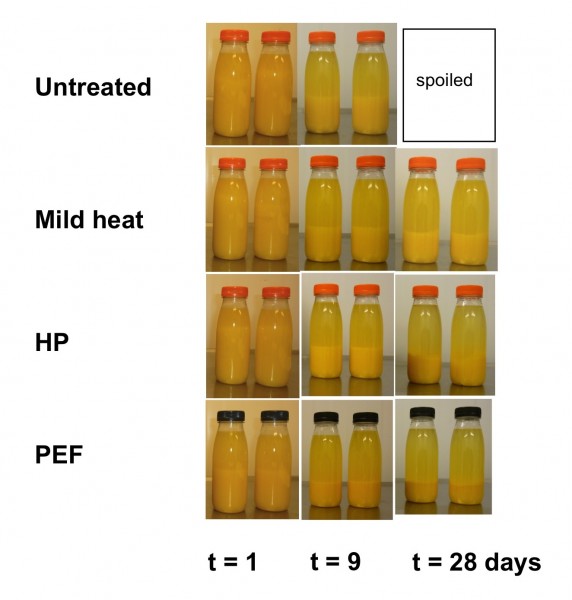

Figure 1 : Photographs of untreated and HP, PEF and heat pasteurised orange juices during shelf life
A wide range of quality attributes were determined directly after processing and during shelf life including pH, colour, total soluble solids, dry matter content and viscosity. Different components were determined including vitamins (ascorbic acid and dehydro – ascorbic acid), sugars, acids and carotenoids; details will be published in peer-review journals later this year. Figure 2, however, gives an example of this work and shows the effect of processing and shelf life on ascorbic acid. The results reveal that only minor changes were observed at the process conditions selected.
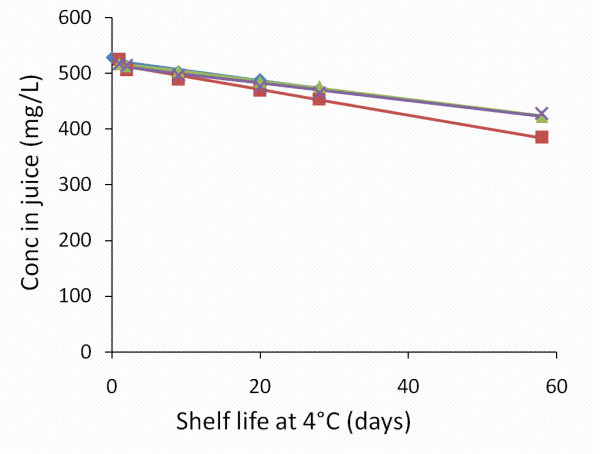

Figure 2 L Ascorbic acid in orange juices directly after processing (untreated (◊), high pressure (Δ), pulsed electric field (X), and heat treatment (■)) and during shelf life
In addition to the chemical, biochemical and physical analyses, sensory evaluation was carried out by trained experts. These experts evaluated the juices directly after processing and during shelf life. After the second evaluation day (nine days), the untreated juices were no longer evaluated because of safety restrictions. Frozen samples were used as an alternative to represent the fresh sample. Figure 3 gives an example of the qualification of samples on the first evaluation day, showing no major differences between the samples. During storage, only minor (non significant) differences in odour were observed.
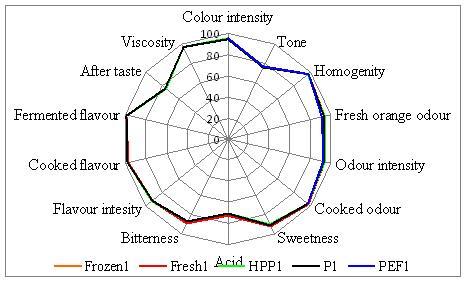

Figure 3 Sensorial evaluation of orange juices directly after processing by trained experts
Acceptance and liking of these juices was also examined by a repeated exposure test. Consumer acceptance was evaluated by observation in the Restaurant of the Future (Figure 4) at Wageningen UR. This facility allows consumer behaviour to be studied in ‘real life’ situations. Juices were offered to regular users of this facility and their buying behaviour was monitored. Almost no differences were observed in buying behaviour between the treatments; most people tasted one of the juices once and then returned to their previous habit.


Figure 4 Restaurant of the Future (Wageningen UR) used for the study of consumer acceptance of orange juice
Conclusions
n general, this integrated demonstration showed that both pulsed electric field treatment and high pressure processing can extend the shelf life of fresh orange juice and maintain its fresh characteristics. The fresh juice spoiled between nine and 20 days while the NP-treated juices were unspoiled after two months chilled storage.
For this specific product, both new technologies and mild heat pasteurisation represent alternatives to obtain a mildly processed product with a longer shelf life under refrigerated conditions. It has yet to be shown whether industrial processing conditions after scaling up would lead to the same result.
The thermal pasteurisation conditions chosen in this study are very mild and were carried out as an HTST process followed by aseptic filling resulting in minimal impact on juice quality. Although the shelf life of the heat pasteurised samples was two months, the temperature-time combination selected should be regarded as insufficient since heat penetration is limited in a large-scale heat pasteuriser, especially with more viscous products. These effects do not occur for NP since the critical process parameters are, in principal at least, not based on a temperaturetime combination.
The current study proves that the comparison of technologies on specific products needs careful selection of critical processing conditions based on the principle of equivalence. At the same time, the study proves that with such careful selection and improvement in conventional processing, better products can be achieved.
Acknowledgement
As this is an integrated demonstration, many partners and co-workers have contributed to this task. We would like to thank everyone who contributed for their efforts and enthusiasm.
About the Authors
Ariette Matser
Ariette Matser is currently Senior Scientist – Mild Preservation and Novel Technologies and Programme Coordinator – Mild Preservation within Food & Biobased Research of Wageningen UR. She studied Food Science at Wageningen UR. Her primary research activities include novel technologies and food safety and quality, preservation and processing of food products and effects on food quality, and food structure and the effects of processing on this. Management activities are involvement in (inter) national R&D projects, project acquisition and coordination of activities on novel technologies. She is Project Coordinator of the European project NovelQ.
Hennie Mastwijk
Hennie Mastwijk is a senior scientist at Wageningen UR Food & Biobased Research, The Netherlands (www.wur.nl). He is involved in the development of equipment and food products through electronic based mild preservation processes.
Diána Bánáti
Diána has been Director General of the Central Food Research Insitute since 2000. She is a member and representative for several inter – national organisations (including the European Food Safety Authority, FAO/WHO Codex, International Life Sciences Institute). Her research avtivites focus on issues related to food science, with special regard to her main fields of interest: food safety, consumer sciences, food policy and agricultural-food ethics. She is largely involved in national R&D progreammes on food safey and international EU support research projects focusing on food safety, new technologies and risk management.
Liesbeth Vervoort
Liesbeth Vervoort obtained a Bioengineer Master’s degree in Chemistry, Major Food Technology, at the Katholieke Universiteit Leuven in 2007. Currently, Liesbeth is working as a PhD student at the Laboratory of Food Technology, Katholieke Universiteit Leuven, performing research on intrinsic and extrinsic indicators for process impact and uniformity of high pressure processing. Her work is part of the integrated European project NovelQ (FP6-CT-2006-015710).
Marc Hendrickx
Marc Hendrickx is Senior Professor in Food Technology at Katholieke Universiteit Leuven. He is Chairman of the Centre for Food and Microbial Technology, Head of the Laboratory of Food Technology (LFT), Member of the Board of LFoRCe (the Leuven Food and Nutrition Research Center) and co-director of the InterUniversity Program in Food Technology (IUPFOOD). Currently, he is a member of the management board of NovelQ, Healthy Structuring and High Tech Europe. He is co-author of over 260 papers in peer review journals and over 200 contributions to international conferences and workshops. His research activities focus on the effect of food processing and preservation technologies on food functional properties.
Issue
Related organisations
Related people
Ariette Matser, Diana Banati, Hennie Mastwijk, Marc Hendrickx





i really need this for my seminar