Tools for safe food
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 31 January 2005 | Geraldine Duffy and Terese Catarame, Teagasc, The National Food Centre, Ireland | No comments yet
The microbiological analysis of food has an important role in assessment of the quality and safety of foods. There is a direct relationship between bacterial numbers and product shelf life as growth of bacteria can result in organoleptic changes in the food, including off-colours and off-odours, rendering it unacceptable to the consumer.
The presence of pathogenic microorganisms on foods (Salmonella spp, Campylobacter, Staphyloccocus aureus, Listeria monocytogenes, E. coli O157:H7 etc.) poses the threat of food poisoning and recent publicity concerning food related health scares have increased consumer concerns regarding food safety. Owing to the economic implications and loss of goodwill associated with a food poisoning incident, food manufacturers recognise the necessity to provide the assurance on food safety that consumers demand. This is achieved by the implementation of food safety management systems such as Hazard Analysis Critical Control Points (HACCP) as well as by testing foods (raw material and end products) to ensure they conform to set microbiological criteria for certain microbial pathogens. These criteria may be set by regulatory authorities or by the customer (often the retailer).
The microbiological analysis of food has an important role in assessment of the quality and safety of foods. There is a direct relationship between bacterial numbers and product shelf life as growth of bacteria can result in organoleptic changes in the food, including off-colours and off-odours, rendering it unacceptable to the consumer. The presence of pathogenic microorganisms on foods (Salmonella spp, Campylobacter, Staphyloccocus aureus, Listeria monocytogenes, E. coli O157:H7 etc.) poses the threat of food poisoning and recent publicity concerning food related health scares have increased consumer concerns regarding food safety. Owing to the economic implications and loss of goodwill associated with a food poisoning incident, food manufacturers recognise the necessity to provide the assurance on food safety that consumers demand. This is achieved by the implementation of food safety management systems such as Hazard Analysis Critical Control Points (HACCP) as well as by testing foods (raw material and end products) to ensure they conform to set microbiological criteria for certain microbial pathogens. These criteria may be set by regulatory authorities or by the customer (often the retailer).
The microbiological analysis of food has an important role in assessment of the quality and safety of foods. There is a direct relationship between bacterial numbers and product shelf life as growth of bacteria can result in organoleptic changes in the food, including off-colours and off-odours, rendering it unacceptable to the consumer.
The presence of pathogenic microorganisms on foods (Salmonella spp, Campylobacter, Staphyloccocus aureus, Listeria monocytogenes, E. coli O157:H7 etc.) poses the threat of food poisoning and recent publicity concerning food related health scares have increased consumer concerns regarding food safety. Owing to the economic implications and loss of goodwill associated with a food poisoning incident, food manufacturers recognise the necessity to provide the assurance on food safety that consumers demand. This is achieved by the implementation of food safety management systems such as Hazard Analysis Critical Control Points (HACCP) as well as by testing foods (raw material and end products) to ensure they conform to set microbiological criteria for certain microbial pathogens. These criteria may be set by regulatory authorities or by the customer (often the retailer).
Traditional methods for the detection of bacteria in foods rely on culturing the bacteria onto agar plates. These traditional methods are very time consuming, taking three days to determine a total viable count and five to seven days to detect specific pathogenic bacteria. Many of the rapid methods currently available are largely unsuitable for use in industrial based laboratories. They lack sensitivity, are expensive and complex to perform, often requiring specialised personnel and significant capital expenditure. The absence of rapid cost effective methods for bacterial detection poses particular difficulties for food items with a short shelf-life and in the monitoring of Critical Control Points (CCPs) in HACCP management systems. This means that there is now a particular need for rapid microbial methods.
In food analysis, rapid methods still lack sufficient sensitivity and specificity for direct testing. Hence, all current methods need at least one enrichment step, involving incubation of at least six – and often up to 48 – hours to increase numbers of the target pathogen to a detectable level. An ideal rapid method should yield a result in a much shorter period than the conventional cutural method; be cost effective; user friendly and preferably not require a major capital investment or highly specialised skills to operate. In addition, such methods must have high sensitivity, high specificity, high precision (repeatability) and be validated against the gold standard cultural test. Currently, no method exists that will fulfil all such requirements.
Approaches to rapid detection of pathogens have been developed based on one of two approaches described below: immunological or molecular methods.
Immunological methods
Immunological methods are based on a reaction between a specific antigen on the target microorganism and a complementary antibody. In order to facilitate detection an antibody label is employed. This may be an enzyme (described below) or a radioactive dye (though these are generally not used in detection of food borne pathogens). A fluorescent dye may be employed as part of a microscope based test in which the microbial cells are stained with a fluorescent labelled antibody and then identified by microscopy or image analysis (Figure 1).
Immunomagnetic separation
The separation of a specific microbial pathogen from a complex matrix, such as a culture enriched food sample which also contains a large and diverse background flora, is one of the biggest challenges in the detection of food-borne pathogens. One approach to this problem is the use of immunomagnetic separation (IMS).
In this technique, immunomagnetic particles coated with antibodies specific to the target pathogen are suspended in an aliquot of enriched food. The particles with bound target cells are separated from the suspension using a magnetic particle separator; the remaining suspension is removed and magnetic particles are washed several times. In comparison with direct plating from enrichment broth, IMS is 100-fold more sensitive for recovery of a particular pathogen. IMS is commercially available for a range of pathogens, including Listeria monocytogenes, Salmonella and E. coli O157, 026 and 0111. The technique is compatible with subsequent detection by cultural, immunological or molecular methods. It is commercially available as part of an automated ELISA system (see below).
Enzyme immunoassay
Immunoassays incorporating enzyme labelled antibodies are the most commonly used type of immunoassay for the detection of pathogens. There are many possible enzyme linked immunoabsorbent assay (ELISA) formats but the ‘sandwich’ assay is most often used in commercially available tests. An antibody bound to a solid surface that may be a micro-titre plate, plastic strip, dip stick, capillary migration system etc. acts to capture the antigen (on the target microorganism) (Figure 2). The second antibody, conjugated with an enzyme, binds to the captured antigen and finally an appropriate substrate is added to give a visible colour change or a measurable change in fluorescence that is deciphered by a fluorescent reader. ELISA detection methods are amenable to automation; can handle high sample throughputs and are useful for screening large numbers of samples. Most commercial ELISA methods for food pathogens require 105-106 cfu per ml of target cell in the enriched food and require a 24-48h enrichment period prior to applying the test.
Molecular methods
The technology of bacterial detection has advanced significantly in recent years and considerable efforts have been focused on the development of genetic based techniques for the detection and characterisation of pathogens in foods. These are based on the detection of a specific piece of genetic material (a specific sequence of nucleic acids i.e. DNA or RNA) that is unique to the target organism and, as such, they are highly specific.
Nucleic acid hybridisation
Nucleic acid hybridisation involves binding a prepared DNA probe (single stranded) with a complementary target DNA sequence (single strand) in the organism being sought. DNA is extracted from the test organism and the double strands of DNA are separated. The DNA probe is then added and if DNA complementary to the probe is present, the fragments hybridise (bind) to form a double helix. The hybridised product can be directly detected if the probe is labelled with a radioactive or fluorescent dye or, alternatively, it can be detected indirectly using an enzyme-linked assay. One of the most common techniques employed for hybridisation is to immobilise the target DNA that has been obtained from either a solution (dot blot format) or following separation of the DNA by gel electrophoresis (southern blot format), onto a solid support such as a membrane (usually nylon or nitrocellulose). A labelled DNA probe is subsequently added to facilitate detection. Colony hybridisation involves blotting a membrane filter against colonies formed on a solid agar plate. The DNA is extracted in situ and fixed to the filter. A labelled DNA probe is subsequently applied to detect the target DNA and the reaction may be visualised in an ELISA type assay.
An alternative hybridisation technique is a sandwich assay. This involves the immobilisation of a DNA probe to a membrane, which then serves as a receptor/capture site for hybridisation with target DNA from the test organism. A second labelled receptor probe is then added that increases both the sensitivity and specificity of the technique.
When these techniques are applied to food samples, a prior enrichment step is necessary to increase the number of pathogenic bacteria and the level of target DNA. Hybridisation methods are usually applied to colonies on agar plates and so give highly specific and fast confirmation/identification of the colony on the plate. This is most helpful in situations where the agars used are not highly selective or do not yield a morphologically distinct colony, but as they generally require a prior enrichment and agar culture phase they are not rapid. There are commercially produced hybridisation kits, which combine probes that target ribosomal RNA genes with a colorometric system for detection of the hybridised products. Other commercial systems combine a hybridisation product with a chemiluminescent detection method. Assays are commercially available for the detection of Salmonella spp., Listeria monocytogenes, E. coli and Campylobacter.
Polymerase Chain Reaction (PCR)Nucleic acid based methods, which incorporate an amplification step for the target DNA, are now the most widely used molecular tests – the most popular method being the polymerase chain reaction (PCR). In this technique the DNA is extracted from the organism and the double strands are denatured into single stranded DNA. Short sequence DNA primers are annealed to the complementary DNA target in the organism. The primers are then extended across the target sequence using a heat stable DNA polymerase (usually Taq polymerase, a thermostable and thermoactive enzyme from Thermus aquaticus) in the presence of free deoxynucleoside triphosphates (dNTPs) resulting in a double replication of the starting target material. Multiple repeats of the denaturation, annealing and extension steps result in an exponential increase in the levels of the initial target DNA, thus greatly increasing the sensitivity of the method. These tests are highly specific as they focus on a particular gene unique to the target organism. However, even with the amplification steps in PCR, which significantly enhance the sensitivity of these techniques, the use of an enrichment step is still necessary when these techniques are applied to the detection of pathogens in foods.
In conventional PCR, the amplified product (target gene) is detected by staining ethidium bromide on an electrophoresis gel. However, this is time consuming and cumbersome and recent developments are focusing on the use of automated real time PCR product as an alternative technique to the use of gel electrophoresis.
In real time PCR the DNA probe has a fluorescent dye which, as amplification occurs in the PCR reaction, gives a fluorescent signal which is measurable in real time (Figure 3). The technique employs fluorescent double stranded (ds) DNA, intercalating dyes or sequence specific probes. This method is considerably faster than conventional PCR; less prone to operator error and more convenient as the PCR amplification and detection are all carried out in one machine. A number of real-time PCR instruments are commercially available.
A limitation of the PCR systems is their inability to distinguish between viable and non viable bacteria, though this is somewhat overcome by sample enrichment which increases the numbers of viable cells and target DNA. However, the detection of RNA is considered to be indicative of viability and ongoing research on reverse transcriptase PCR (RT-PCR) is yielding PCR techniques which will detect viable bacteria only. In this technique the enzyme RT converts RNA into DNA molecules – making them targets for PCR amplification. The difficulty with this technique is that isolation of RNA is technically more difficult than DNA and it is also less stable.
Developmental amplification methods
A number of alternative techniques to PCR that allow amplification of nucleic acids are being developed. One such method that is showing particular promise is nucleic acid sequence based amplification (NASBA). This is an isothermal amplification system, which selectively amplifies RNA through the concerted action of three enzymes. The NASBA system has been applied to the detection of Campylobacter jejuni, Listeria monocytogenes and Salmonella.
Future developments
A new development that combines semi-conductor technology with molecular biology to build ‘DNA chips’ has the potential to revolutionise molecular microbiology diagnostic techniques. DNA chips consist of very large arrangements (arrays) of oligonucleotides on a solid support. Labelled sample test DNA can be added to the DNA chip or ‘microarray’ and, if complementary DNA is present, hybridisation will occur. The hybridised product can be detected by fluorescence scanning or by using enzyme technology. Using this technology, it may in the future be possible to detect and type a range of pathogenic bacteria using a single detection method. Some of the many problems yet to resolved in the application of this technology are sample preparation, cross-reactions with non target DNA and a need for improved sensitivity.


Figure 1: Micro-titre plate ELISA assay

Figure 2: Loading a gel as part of gel electrophoretic PCR product detection
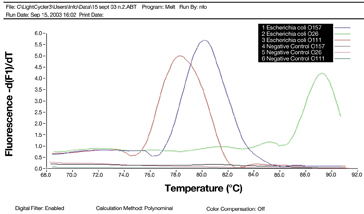

Figure 3: Real time PCR (fluorescence output)
Further Reading
de Boer E, Beumer RR (1999) Methodology for detection and typing of foodborne microorganisms, International Journal of Food Microbiology 15: 50(1-2):119-130.
Fung DY. (2002) Predictions for rapid methods and automation in food microbiology J AOAC Int. 85: 4, 1000-1002.
Rijpens NP, Herman LM. (2002) Molecular methods for identification and detection of bacterial food pathogens J AOAC Int. 85: 4, 984-995
Stevens KA and Jaykus LA (2004) Bacterial separation and concentration from complex sample matrices: a review, Critical Reviews Microbiology 30: 1, 7-24.
Call DR, Borucki MK and Loge FJ. (2003) Detection of bacterial pathogens in environmental samples using DNA microarrays, J Microbiol Methods.53: 2, 235-243.




