Bacterial tolerance – the consequences
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 21 November 2005 | Even Heir and Solveig Langsrud, Matforsk AS, Norwegian Food Research Institute, Norway | No comments yet
Failure in cleaning and disinfection increases the ability of bacteria to survive, adapt and establish in food processing equipment or other environments, with the potential to transfer to food products. The antimicrobial effects of disinfectants depend upon several factors. This article focuses on the properties and mechanisms of bacteria involved in increased tolerance to disinfectants used in the food industry.
Failure in cleaning and disinfection increases the ability of bacteria to survive, adapt and establish in food processing equipment or other environments, with the potential to transfer to food products. The antimicrobial effects of disinfectants depend upon several factors. This article focuses on the properties and mechanisms of bacteria involved in increased tolerance to disinfectants used in the food industry.
Failure in cleaning and disinfection increases the ability of bacteria to survive, adapt and establish in food processing equipment or other environments, with the potential to transfer to food products. The antimicrobial effects of disinfectants depend upon several factors. This article focuses on the properties and mechanisms of bacteria involved in increased tolerance to disinfectants used in the food industry.
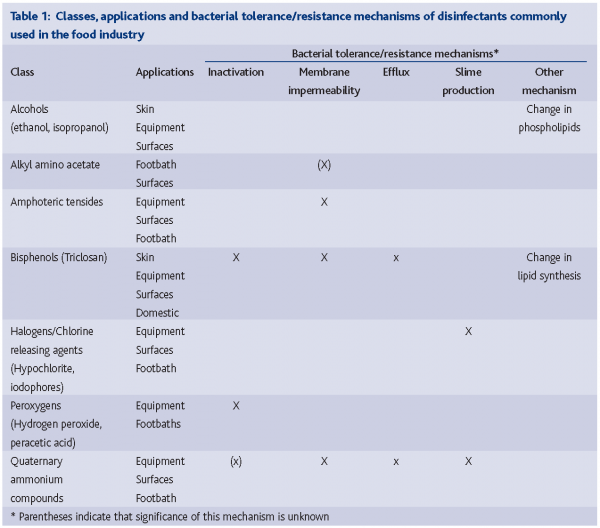
In light of the diversity and adaptive capacity of bacteria, it is not surprising that bacteria possess a wide range of properties and mechanisms to survive in environments where disinfectants are regularly used. Mechanisms of disinfectant action and resistance have gained renewed attention, as connections between disinfectant tolerance and antibiotic resistance have become obvious. To design safe and effective disinfection strategies that prevent bacterial tolerance/ resistance development, knowledge on how bacteria and disinfectants interact under various conditions is essential. Both the beneficial and potential negative effects should be documented. Table 1 presents an overview of classes of disinfectants, their applications, mechanisms of action and reported resistance mechanisms. The article will provide a brief overview of the biological basis for bacterial tolerance to commonly used disinfectants in the food industry and discuss potential consequences of bacterial tolerance to disinfectants.
Bacterial strategies to survive cleaning and disinfection
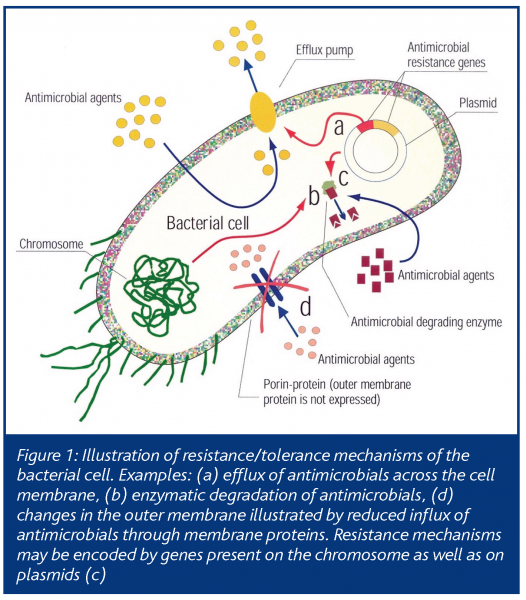
The microflora in food environments represents a high diversity of bacteria that are present in different environments and in various physiological states. Environmental factors affect the physiology of bacteria, their phenotypic properties and, hence, their susceptibility to disinfectants. The variations in disinfectant tolerance are also due to differences in innate and acquired properties of the organisms. These include membrane structure, efflux pumps, the ability to inactivate disinfectants and mutations conferring altered targets sites and differences in expression of protective mechanisms (Figure 1).
Physiology affects disinfectant tolerance
The antimicrobial effect of disinfectants is dependent, at least in part, on the ability of the compound to interact and permeate the cell membrane. The innate properties of the cell membrane differ considerably between bacteria. Bacterial spores have especially complex and rigid membrane structures that are effective barriers against most antibacterials. Gram-negative bacteria are generally more tolerant to disinfectants than gram-positives, mainly due to the relatively impermeable outer membrane of the former. Membrane properties are not static, but vary significantly according to environmental factors. Certain gram-positive bacteria are surrounded by slime, which increases the natural resistance towards many disinfectants. Other bacteria form aggregates when exposed to stressful conditions and this increases their disinfectant tolerance. Under natural conditions, most bacteria are either attached to surfaces or embedded in biofilms. This makes them much more tolerant to disinfectants than bacteria in solution. Biofilms are communities of surface adhered bacteria that are embedded in a self-produced polymeric matrix. The tolerance of biofilm-embedded bacteria to disinfectants is dependent on the biofilm structure and the physiological state of bacteria. Anoxic conditions and nutrient depletion is typical, at least in parts of the biofilms. Such conditions are considered to activate bacterial responses that contribute to increased tolerance to many physical and chemical stresses.
Efflux of antimicrobials
Bacteria contain cell membrane proteins that transport compounds across the membrane. Of special note is the broad substrate specificity of many of these proteins, termed efflux pumps, meaning that they can transport divergent compounds across the membrane. Efflux can provide protection of the bacteria against antibiotics and, in combination with other mechanisms or bacterial phenotypic properties, efflux is also a highly effective and flexible resistance mechanism towards many disinfectants. Bacteria within the genera pseudomonas, for instance, are highly tolerant to many antimicrobials. This property is obtained through a combination of impermeable outer membrane and activity of broad-substrate efflux pumps. Many efflux systems are active when the bacteria are exposed to certain stresses while they are inactive when their function is unnecessary. It has been demonstrated that sub-lethal exposure of certain bacteria to disinfectants or other stresses relevant in food and food production can activate efflux mechanisms. In gram-positive bacteria (e.g. staphylococci), genes encoding efflux pumps are often located on plasmids (extrachromosomal genetic elements) that can be transferred between bacteria. This contributes not only to the effective spread of disinfectant resistance genes, but also to certain antibiotic resistance genes that are frequently co-located on plasmids. Efflux pumps thus effectively contribute to increased resistance/tolerance to a wide spectrum of compounds in many bacteria. Although still debated, recent reports indicate that misuse and repeated sub-lethal exposure to certain disinfectants may contribute to selection of resistant microorganisms. Knowledge on how different physicochemical factors affect the activity of efflux and other resistance mechanisms should be kept in mind in order to avoid, for example, processing and cleaning strategies that stimulate selection and expression of resistance mechanisms.
Degradation of disinfectants
Certain bacteria have the ability to use enzymes to degrade certain disinfectants (e.g. quaternary ammonium compounds and triclosan). Some bacteria have the ability to use disinfectant compounds as a carbon source, thus potentially stimulating growth rather than inhibiting or killing the bacteria. Although of unknown significance, bacteria with the ability to degrade disinfectants contribute to environments with lower concentrations of active compounds that may stimulate survival, adaptation and/or selection of certain bacteria.
Genetic mutations
Exposure to antimicrobials may provoke genetic mutations. The effect of triclosan exposure to various bacteria has been extensively studied since the first reports of the antibacterial mechanisms of triclosan. This indicated triclosan, at least at sub-lethal concentrations, to exert its antibacterial action on a single bacterial enzyme (enoyl reductase; involved in bacterial lipid synthesis). Triclosan thus acted more as an antibiotic than as a multi-target disinfectant. Notably, sub-lethal concentrations of triclosan can select for mutations in the gene encoding this enzyme, making triclosan inactive and thus increase the tolerance of triclosan among bacteria. Of significant concern was the observation that this enzyme is also the target for the antitubercular agent, isoniazide. However, triclosan and isoniazide seem to have distinct interactions with this target. It is likely that other disinfectants also select mutations within specific targets, some of which may be common to other antimicrobials. Chromosomal mutations also lead to changes in the specificity and regulations of various efflux systems, thus contributing to increased resistance to antimicrobials. Strategies to avoid repeated sub-lethal exposures to disinfectants should be emphasised to reduce the risk for development and selection of bacterial mutants with increased tolerance to antimicrobials.
Bacterial resistance to disinfectants – what are the consequences?
Disinfectants used at recommended user concentrations, generally affect several bacterial targets. This has been considered to limit the ability of bacteria to develop tolerance/resistance to disinfectants and is used as an argument to allow widespread use of disinfectants. However, in practice, concentration gradients of disinfectants will be formed. The bactericidal activity of certain commercially available surface materials used in the food industry is not adequate and allows growth of many bacteria. Thus, at least some bacteria will frequently be exposed to sub-lethal concentrations of disinfectants. This adaptation (‘training’) to survive in environments with disinfectants could contribute to selection and survival of specific bacteria that affect shelf life and microbial safety of involved food products.
Of potential significance, it has been demonstrated that bacteria adapted to growth in increasing concentrations of certain disinfectants also become increasingly resistant to clinically significant antibiotics. It is also clear that certain disinfectants on the one hand (e.g. triclosan) and antibiotics on the other have similar effects on bacteria. However, the capacity of bacteria to adapt to disinfectants is not general, but dependent on a number of factors where the antibacterial mechanisms of the disinfectant, as well as the properties of the microorganism or microflora, are the most obvious. One example of selection or adaptation is the isolation of coliforms resistant to tenside based disinfectant from disinfecting footbaths. In these baths the active concentration of disinfectant will gradually decrease during the working day, due to contamination with dirt. Higher frequency, or disinfectant tolerance, of bacteria isolated after disinfection compared to bacteria from other sources confirms that disinfection may lead to selection of resistant strains and/or adaptation to disinfectants. Use of certain disinfectants may co-select for antibiotic resistance since disinfectant resistance genes may be located adjacent to antibiotic resistance genes in both gram-positive and gram-negative bacteria.
Bacterial exposure to certain disinfectants stimulates bacterial attachment to surfaces. There is limited knowledge on how environmental factors affect surface attachment and biofilm formation. This has potentially profound implications for the production of safe food. The ability of many bacteria to adapt to certain disinfectants should be kept in mind and one should focus on eliminating conditions and factors that promote adaptation to disinfectants.
To date, most studies and conclusions on bacterial resistance are based on laboratory investigations of single, culturable bacterial strains. Consequently, while disinfectant use may lead to antibiotic resistance in the laboratory, it does not necessarily equate to the development of such resistance in natural, highly complex environments. More information is needed on the long-term effects of widespread use of disinfectants with regard to bacterial ecology, resistance development and environmental effects. Until more knowledge is obtained, use of disinfectants should be restricted to areas and products where they have a documented antimicrobial effect and where these positive effects far outweigh potential negative effects.
How to avoid resistance in practice?
Based on scientific studies and our experience, it is possible to make some recommendations regarding measures to avoid resistance:
- Choose an effective disinfectant
Classes of disinfectants differ in their properties, for example, targets and modes of action and in their ability to inhibit and kill bacteria under various conditions. To choose the cheapest disinfectant may be tempting, but the price is often related to the concentration of active components and the competence of the supporter.
- Disinfect at optimal conditions
Never disinfect a dirty surface and use the recommended concentration, temperature and exposure time. The efficacy depends on exposure time, temperature and concentration and is usually reduced by organic matter, whatever the supplier claims. Recommended user concentrations of disinfectants should be applied to avoid possible selection and bacterial adaptation to disinfectants.
- Rinse thoroughly after disinfection
Exposure to sub-lethal concentrations of disinfectants may allow bacteria to adapt. This can increase the ability of bacteria to grow and survive in higher concentrations of disinfectants and to develop cross-resistance to antibiotics.
- Alternate between different disinfectants
Using another disinfectant once a week or alternating every second week will probably kill resistant bacteria. It is important to choose disinfectants with completely different mechanisms of action.


References
Gilbert, P., and A. J. McBain. 2003. Potential impact of increased use of biocides in consumer products on prevalence of antibiotic resistance. Clin. Microbiol. Rev. 16:189-208.
Heir, E., G. Sundheim, and A. L. Holck. 1999. Identification and characterization of quaternary ammonium compound resistant staphylococci from the food industry. Int.J.Food Microbiol. 48:211-219.
Heir, E., G. Sundheim, and A. L. Holck. 1995. Resistance to quaternary ammonium compounds in Staphylococcus spp. isolated from the food industry and nucleotide sequence of the resistance plasmid pST827. J.Appl. Bacteriol. 79:149-156.
Langsrud, S., M. S. Sidhu, E. Heir, and A. L. Holck. 2003. Bacterial disinfectant resistance – a challenge for the food industry. Int.Biodeterior.Biodegrad. 51:283-290.
Langsrud, S., G. Sundheim, and A. L. Holck. 2004. Cross-resistance to antibiotics of Escherichia coli adapted to benzalkonium chloride or exposed to stress-inducers. J.Appl.Microbiol. 96:201-208.
McDonnell, G., and A. D. Russell. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin.Microbiol.Rev. 12:147-179.
Moretro, T., L. Hermansen, A. L. Holck, M. S. Sidhu, K. Rudi, and S. Langsrud. 2003. Biofilm formation and the presence of the intercellular adhesion locus ica, among staphylococci from food and food processing environments. Appl. Environment. Microbiol. 69:5648-5655.
Russell, A. D. 2004. Bacterial adaptation and resistance to antiseptics, disinfectants and preservatives is not a new phenomenon. J. Hosp. Infect. 57:97-104.
Schweizer, H. P. 2001. Triclosan: a widely used biocide and its link to antibiotics. FEMS Microbiol.Lett. 202:1-7.
Sidhu, M. S., E. Heir, H. Sorum, and A. Holck. 2001. Genetic linkage between resistance to quaternary ammonium compounds and beta-lactam antibiotics in food-related Staphylococcus spp. Microb Drug Resist 7:363-71.




