Cooling conditions for compound coatings
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 7 March 2007 | NF | No comments yet
Recent moves away from using partially hydrogenated fats (owing to their trans fatty acid content) have had a major impact on the use of compound coatings in confectionery products. Historically, these have fallen into two main types:
Recent moves away from using partially hydrogenated fats (owing to their trans fatty acid content) have had a major impact on the use of compound coatings in confectionery products. Historically, these have fallen into two main types:
Recent moves away from using partially hydrogenated fats (owing to their trans fatty acid content) have had a major impact on the use of compound coatings in confectionery products. Historically, these have fallen into two main types:
- Non-lauric cocoa butter replacers (CBR) such as Loders Croklaan’s Couva range based on partially hydrogenated and fractionated oils such as palm oil, soyabean oil, cottonseed oil or rapeseed oil
- Lauric cocoa butter substitutes (CBS) such as Loders Croklaan’s CLSP range based on palm kernel oil after either hydrogenation or fractionation or both
The move away from trans fats has greatly affected the use of the traditional non-lauric CBR and has also had some impact on some of those CBS produced by hydrogenation, i.e. HPKO. In terms of what to use in place of high-trans, non-lauric CBR manufacturers have basically had three options:
- Low-trans non-lauric CBR
- Trans-free CBS
- Supercoating
We have not considered the move to a low-trans CBR as being a viable option because (a) there is still some trans fatty acid present which would then limit its use in some countries, and (b) the product still needs to be labelled as ‘hydrogenated’ which is a problem to many consumers. This leaves only the options of a trans-free CBS or a supercoating.
A supercoating is a coating similar to chocolate but instead of the added fat in the recipe being cocoa butter it is a cocoa butter equivalent. This means that, although it cannot be labelled as chocolate, the sensory characteristics of the coating are very similar to those of chocolate. Indeed, its similarity to chocolate is such that a supercoating also needs to be tempered. Non-lauric CBR coatings are non-temper so changing to a supercoating is a big change for many manufacturers to make. Nevertheless this has often been the preferred solution for much of the market. In this article, however, we are concentrating on the other option of changing to a lauric-based CBS coating and the implications, particularly on crystallisation, of making such a change.
The lauric fats used in CBS coatings have effectively no compatibility with cocoa butter and so a lower cocoa fat recipe needs to be used for such coatings compared to the original non-lauric CBR coatings. This may then mean a certain compromise on flavour.
The way in which the two types of fat crystallise also differs. So, for example, a coating based on non-lauric CBR will crystallise at a different rate or at a different optimum temperature than one based on CBS. Not only that, but changing the fat can also have an effect on the relative hardness of the coatings. To give a more scientific basis to these differences – and especially to the reasons for these differences – some fundamental research on their thermal properties, crystallisation rates and hardness has been carried out. This work, funded by Loders Croklaan, was performed at Ghent University, Belgium by Imogen Foubert and her team1. This article takes the result of that work and relates it in a practical sense to the change from a non-lauric CBR coating to a CBS coating.
Comparison of Non-Lauric and Lauric based coatings
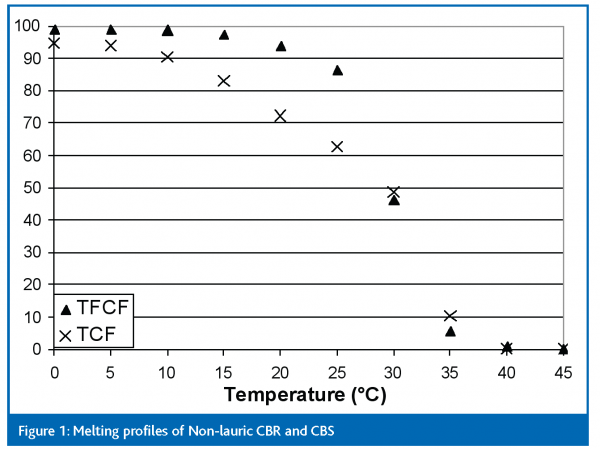
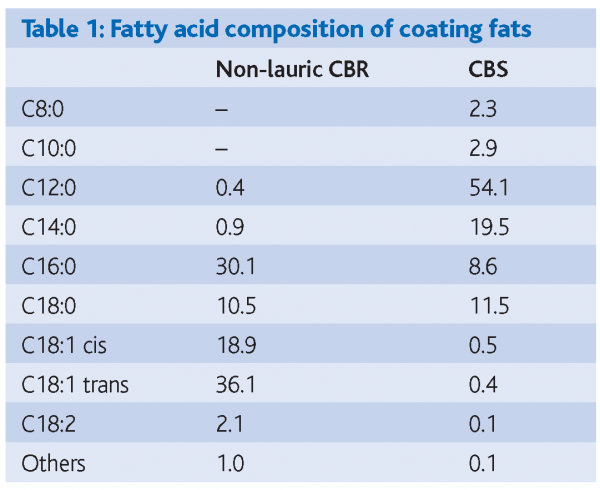
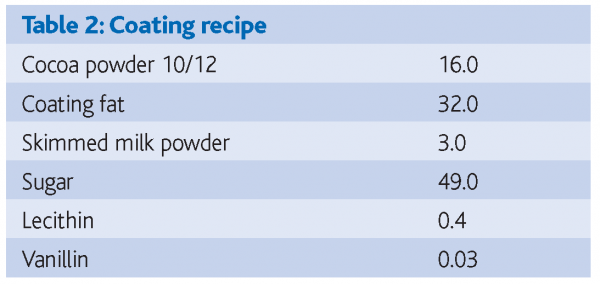
Two coating fats – a non-lauric CBR based on partially hydrogenated palm oil and a CBS based on fully hydrogenated, fractionated palm kernel oil – together with coatings made from these two fats – formed the basis of the study. Because the CBS was a fully hydrogenated fat it contained effectively no trans fatty acids, as can be seen from Table 1. Coatings from both oils were made to the same recipe (Table 2). The recipe contained 4.8 per cent cocoa butter in the fat phase, just within the normal limit of 5 per cent in recipes containing lauric fats. The melting profiles of the two fats are shown in Figure 1.
Rate of crystallisation
When the fats or coatings containing the fats are crystallised then the solid fat content at the cooling temperature may be considered to be the ‘target’ level for equilibrium during cooling. This level is, however, not always achieved either because crystallisation is slow or because further post-crystallisation and post-hardening occurs during storage. Despite this, it is still possible to achieve an equilibrium solid fat level during cooling (which may be lower than the solid fat content shown in Figure 1 for that cooling temperature). What is important is that (a) that equilibrium should be attained in a fairly short time to maximise cooling tunnel throughput and (b) the equilibrium solid fat content should be high enough for the coating to dry to the touch and able to be immediately wrapped on exiting the tunnel.
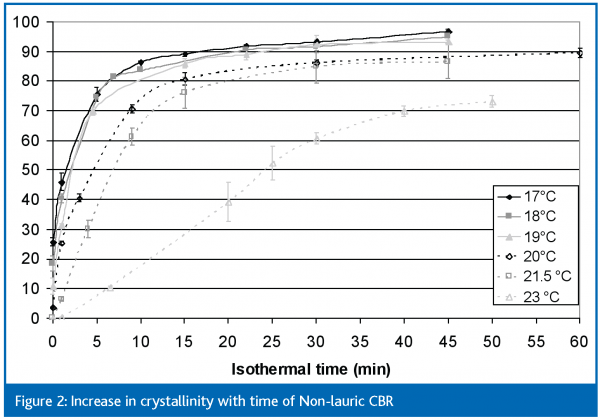
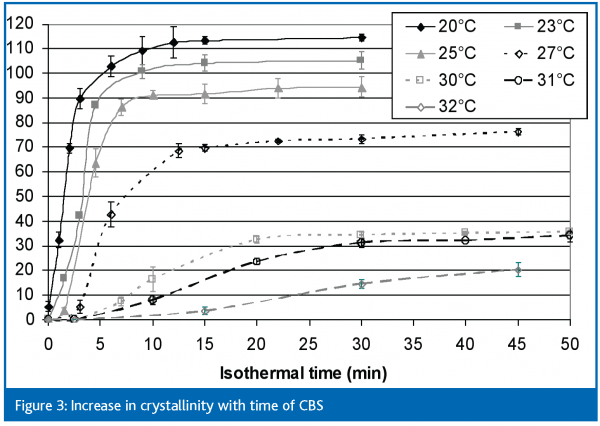
While passing coated products under different cooling conditions through a cooling tunnel and examining them at various time intervals is clearly the best approach in defining optimal cooling conditions, it is also quite time consuming. A simpler approach, at least for initial screening, is to look at the crystallisation of the fats themselves using, for example, differential scanning calorimetry (DSC). This uses a very small sample which can be held at specific temperatures. The melting enthalpy, which is a measure of crystallinity, can be used to show rates of crystallisation (although this is not a measure of absolute solid fat content). The increases in melting enthalpies of both coating fats held at different temperatures are shown in Figures 2 (non-lauric CBR) and 3 (CBS).
The first thing that is clear from these two diagrams is how much more quickly CBS crystallises compared to non-lauric CBR. Cooling temperatures below 20°C are needed to reach equilibrium within 15-30 minutes with non-lauric CBR, whereas equilibrium is reached in shorter times and at higher temperatures with CBS. This is, in itself, an interesting observation because, although it is well known that lauric fats crystallise more quickly than do non-lauric fats, lauric-based coatings are often cooled at quite low tunnel temperatures (-8°C). Secondly, although an equilibrium is reached within approximately 30 minutes when the CBS coating is cooled at 30°C, the solid fat content at that point would be insufficient to allow the product to be wrapped, whereas the equilibrium reached within approximately 15 minutes at even 25°C would equate to quite a dry coating. This does not mean, however, that lauric coatings can be wrapped after 15 minutes in a cooling tunnel at 25°C because (a) these measurements were made on the fat alone and (b) on a very small amount of fat which will, of course, then crystallise quite quickly.
Polymorphism and crystal structure
Before moving on to look at the two fats in coatings it is worth commenting on their polymorphism. It has often been assumed that coating fats such as these crystallise directly into the β’ polymorphic form and therefore tempering of any kind is unnecessary. To a large extent this is true, but there is some evidence that, at low crystallisation temperatures, an initial α form is produced. From the DSC information these temperatures are below 23°C for the non-lauric CBR coating and below 20°C for the CBS coating. The α form is, however, quite transient and rapidly transforms to β’.
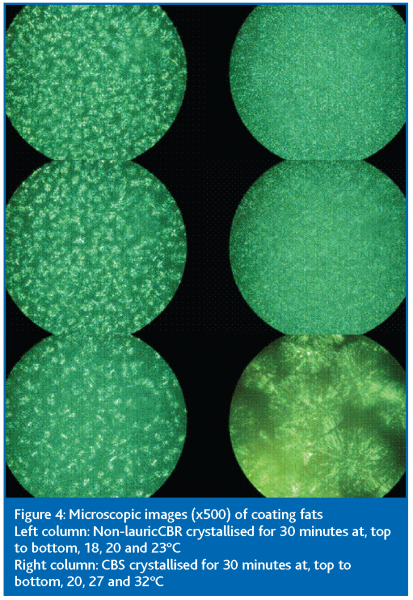
As has already been mentioned the rate of crystallisation of the two fats is very much temperature-dependent. It is well-known that crystal size and structure is linked to crystallisation rate, so it is not surprising to find that these characteristics are also temperature-dependent. Figure 4 shows microscope photographs of both fats crystallised at different temperatures. The number of crystals decreases as the crystallisation temperature of non-lauric CBR increases but there is little effect on the crystal size. There is, however, a clear difference in the crystals of CBS at about 30°C where less fat has crystallised and bigger crystals were produced.
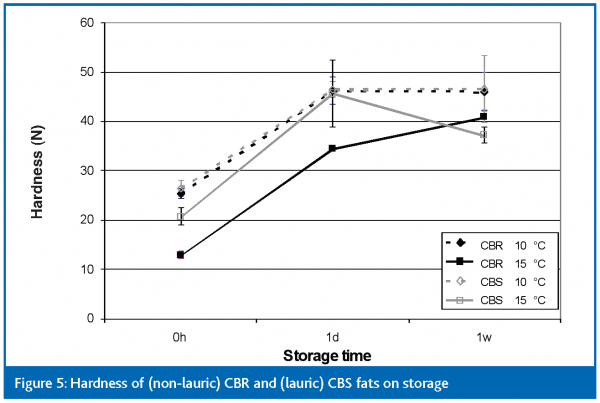
Hardness
The hardness of the two coating fats was studied by cooling 20ml of fat at 10°C and 15°C for 30 minutes. After crystallising in this way the hardness was measured by means of penetrometry, immediately after cooling as well as after 1 day and 7 days storage at approximately 20°C (Figure 5). The non-lauric CBR fat exhibits the type of post-hardening that is often found with partially hydrogenated coating fats of this type, having a large increase in hardness in the first day of storage followed by a lesser or no increase thereafter. The CBS fat crystallised at 10°C follows almost exactly the changes in hardness found with non-lauric CBR crystallised at that temperature. This is somewhat surprising because (a) non-lauric CBR is less crystallised than CBS immediately after cooling, and (b) CBS showed a smaller crystal size than non-lauric CBR. Both of these would lead us to expect the non-lauric CBR fat to be softer at this point than the CBS fat. The CBS fat crystallised at 15°C, however, shows a decrease in hardness between 1 day’s and 7 days’ storage. This may be due to a change in the crystal structure but this has not been confirmed microscopically.
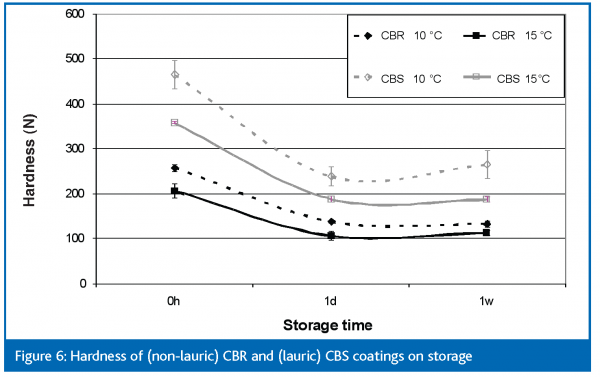
Moving from the fats to the coatings containing these fats gives us an entirely different result (Figure 6). In both cases, the hardness of the coatings decreases markedly during the first day’s storage and then more or less levels off. This is completely opposite to what was seen with the fats themselves. There is no decrease in the amount of crystallisation, nor is there a change in polymorphism to account for this decrease. The only explanation, therefore, is that a change in the crystal structure is occurring. It is generally well known that lauric based coatings have excellent hardness and snap whereas non-lauric CBRs tend to be softer and more malleable making them ideal for ‘cuttable’ cake coatings, for example. These differences were seen here with the hardness of the CBS coating crystallised at 10°C being almost twice that of the non-lauric CBR coating crystallised at the same temperature. What is more surprising is that no such differences were seen between the two fats cooled under the same conditions. This could be a result of the shorter chain triglycerides in CBS being able to form a tighter, firmer network in the presence of matrix components and that this results in a higher hardness.
Summary
Replacing a partially hydrogenated non-lauric CBR coating with one based on a lauric CBS can have quite significant implications for both the way the product needs to be cooled and for the end coating itself. The main aspects to bear in mind are that the CBS system will crystallise more quickly meaning that cooling tunnel conditions may be more flexible than first thought and that the final CBS coating will be significantly harder than the corresponding non-lauric CBR coating. This could have implications for the use of the coating, especially if the original use of the coating was on bakery products where a slightly softer coating might be more preferable.








Reference
- Foubert I, Vereecken J, Smith KW, Dewettinck K – ‘Relationship between crystallisation behaviour, microstructure and macroscopic properties in trans containing and trans free coating fats and coatings’ – Journal of Agricultural and Food Chemistry, 54(19), 7256-7262 (2006)




