Natural antimicrobials derived from the cultivated hop (Humulus lupulusL.)
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 7 July 2011 | Paul Hughes, School of Life Sciences, Heriot-Watt University | No comments yet
To many, consumers and non-consumers alike, there is an understanding that hops are an essential ingredient for the production of beer. However, the history of hop usage for beer production is not as venerable as beer production itself. The hop plant, Humulus lupulus L., was mentioned as early as the first century AD by Pliny the Elder in his prolific ‘Natural History’ (This 37-volume work is the only surviving example of the writings of Pliny the Elder, as he and all his other works perished during the Vesuvius eruption of August AD 79) as being a salad ingredient. However, it was only in the 12th Century that the value of hops for flavouring and preservation of beverages seems to have been recognised. Their use in beer is probably of German origin and hop gardens are often mentioned in German statutes of the 13th Century, when the land dedicated to hop growing must have slowly increased. To this day, Germany is one of the principal hop growing regions in the world.
To many, consumers and non-consumers alike, there is an understanding that hops are an essential ingredient for the production of beer. However, the history of hop usage for beer production is not as venerable as beer production itself. The hop plant, Humulus lupulus L., was mentioned as early as the first century AD by Pliny the Elder in his prolific ‘Natural History’ (This 37-volume work is the only surviving example of the writings of Pliny the Elder, as he and all his other works perished during the Vesuvius eruption of August AD 79) as being a salad ingredient. However, it was only in the 12th Century that the value of hops for flavouring and preservation of beverages seems to have been recognised. Their use in beer is probably of German origin and hop gardens are often mentioned in German statutes of the 13th Century, when the land dedicated to hop growing must have slowly increased. To this day, Germany is one of the principal hop growing regions in the world.
To many, consumers and non-consumers alike, there is an understanding that hops are an essential ingredient for the production of beer. However, the history of hop usage for beer production is not as venerable as beer production itself. The hop plant, Humulus lupulus L., was mentioned as early as the first century AD by Pliny the Elder in his prolific ‘Natural History’ (This 37-volume work is the only surviving example of the writings of Pliny the Elder, as he and all his other works perished during the Vesuvius eruption of August AD 79) as being a salad ingredient. However, it was only in the 12th Century that the value of hops for flavouring and preservation of beverages seems to have been recognised. Their use in beer is probably of German origin and hop gardens are often mentioned in German statutes of the 13th Century, when the land dedicated to hop growing must have slowly increased. To this day, Germany is one of the principal hop growing regions in the world.
During the 14th Century, hop cultivation was developed in the Netherlands, especially in Flanders, and it is likely that their use in England may have been due to immigrant Dutch weavers. However, beer (i.e. hopped ale) was considered to be a foreign drink, whilst the conventional ale (i.e. unhopped) continued to be popular. Nonetheless, the use of hops gave brewers the advantage of producing a final product with appreciably longer shelf-life before it spoiled, and soon plots of land were being cultivated in England – particularly in Kent – to produce the hops necessary. Commercial cultivation did not really begin in earnest until the early part of the 16th Century, and indeed the state-of-the-art at that time was encapsulated in Reynold Scot’s book, ‘A Perfite Hoppe Garden’, the first text to describe hop growing in England, which was published in three editions in 1574, 1576 and 1578.
It is no exaggeration to say that hops allowed the Tudor brewers and their successors to produce beer for markets further afield. This enabled the development of larger breweries and improved the flexibility of beer production. Indeed, it was common to avoid the production of unhopped ale during the summer months precisely because of its lamentable stability. It should be said that the bitterness that hops contributed to beer was by no means universally popular, but rather it was tolerated by an increasing proportion of beer drinkers, although unhopped ale was still produced up to the 19th Century. However, the use of hops helped to ensure a better quality product (once, or rather if, consumers adjusted to beer bitterness) at the point of consumption. The key question then is why hops are able to retard the spoilage of beer? The reliability of beer as a potable liquid was exemplified by the first epidemiological study, carried out by John Snow in London in the 1850’s1, and it is tempting to rationalise Snow’s observations with the antibacterial properties of hops. However, the relative safety of beer consumption in this case was far more likely to be due to the fact that there is a mandatory boiling stage during beer production which effectively sterilises beer during production. Indeed, hop acids are inactive against Gram-negative bacteria such as the cholera-causing vibrios.
It was not until the beginning of the 20th Century that significant progress was made in the identification of the bacteriostatic agents derived from hops. It was clear from as early as the close of the 19th Century that certain extracts of hops contained these2 although it was some time before the two major hop resin components of hops the α- and β-acids – were unequivocally defined (Figure 1). These two groups of compounds occur in remarkably high quantities in hop cones (for example, some German varieties, such as Hallertau Herkules, average close to 17 per cent (w/w) α -acids as is3 (see for instance www.barthhaasgroup.com)), and indeed are bacteriostatic in their own right4. More precisely the hop resin components are found, together with hop essential oils, in sticky yellow extensions of hop cone ‘leaves’ or bracteoles, which are known as lupulin glands. These are typically 150 – 250 μm in diameter and are the main commercial value of hops (Figure 2). However, the hop resins are poorly soluble in aqueous media and indeed are not the principal bacteriostatic agents in beers. During beer production, hops are traditionally introduced at the so-called wort boiling stage of the process. Here the α -acids undergo a remarkable chemical transformation, forming isomers – the iso- α -acids – which are responsible not only for most of the bacteriostatic properties of beer, but also their bitterness and the property of stable foam formation (Figure 3).
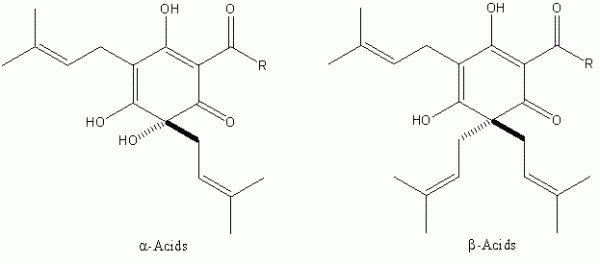

Figure 1The major resin components of Humulus lupulus L. Both classes of compound exhibit bacteriostatic activity against Gram-positive bacteria. The R group represents differences in the biosynthetic pathway of the α- and β-acids, and can be isopropyl (valine origin), isobutyl (leucine origin) and sec-butyl (isoleucine origin). The two former predominate in all hop varieties known.
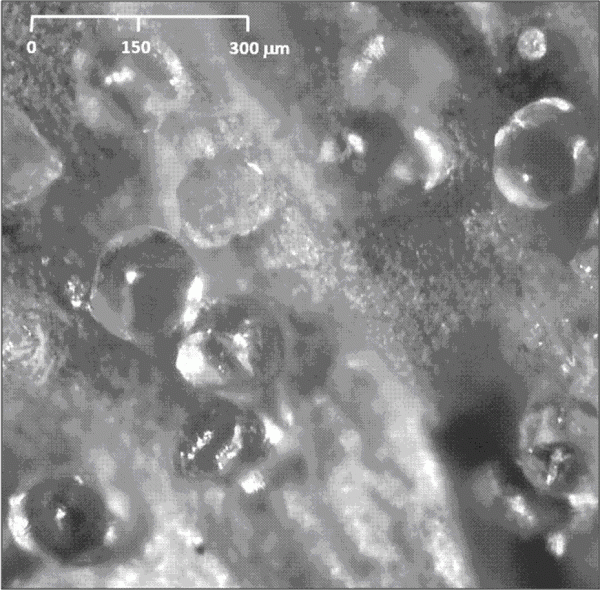

Figure 2 Photomicrograph (40 x magnification) of lupulin glands (mostly roughly spherical structures here), adhering to a supporting hop cone ‘leaf’ or bracteole. The hop resins and essential oils are contained within a bounding structure of a single cell cup covered by a convex extension of the cuticle of the cup cells
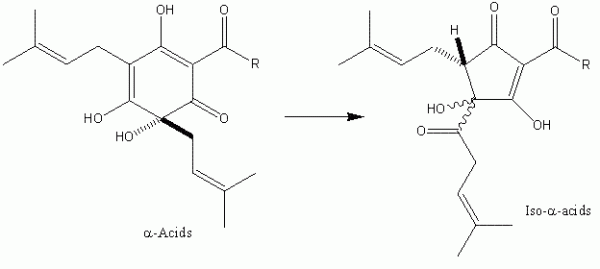

Figure 3 The ring contraction that converts α-acids into iso-α-acids. During traditional beer production this reaction rarely exceeds a 50 per cent yield, but modern hop processors can achieve 90+% yields by optimisation of the isomerisation process.
The three groups of compounds have obvious structural features in common:
- They bear β-diketone moieties, which suggests that they are able to chelate metal cations
- There are a large number of tautomeric variants possible for each (The determi – nation of the pKa values of all of the hop acid family is complicated by the presence of a range of keto-enol tautomers, each of which is likely to have different pKa values. Thus, for instance, a pKa of 3.1 for the iso-α-acids should be regarded as an approximation for what is, nonetheless, a distinctly acidic compound)
- There are several hydrophobic groups present which impact on aqueous solubility
- The extended tricarbonyl feature provides a convenient UV-chromophore for analytical purposes
The hydrophobicity appears to be a necessary requirement for the bacteriostatic activity, at least of the iso-alpha-acids. If the iso-alpha-acids are exposed to high pH (i.e. > 9), the isohexenoyl side-chain is cleaved to yield the corresponding humulinic acids (Figure 4). These compounds, in contrast to the iso- α -acids do not affect bacterial growth. For that matter, unlike the iso- α -acids, they are also neither foam stabilising nor bitter. Based on work carried out principally by Simpson4,5 a plausible mechanism seems to proceed as follows. The hop compound chelates a metal cation and this relatively hydrophobic species diffuses into the bacterial cell membrane. The hop acid acts as an ionophore, essentially swapping intracellular protons for metal cations, thereby destroying the pH gradient across the cell membrane so that the bacterium eventually starves. Thus the features of metal chelation and appreciable hydrophobicity are required to fulfill this mechanistic rationalisation. Further – more, this mechanism helps to explain the pH dependence of the bacteriostatic potency of the hop acids. They are more potent at lower pH and the potency appears to be exactly in line with predicted levels of undissociated acid that can be determined from the pKa values of the various com-pound groups.
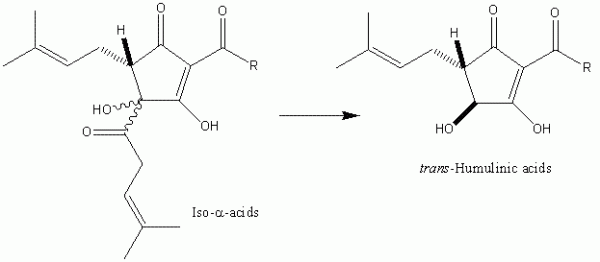

Figure 4 Excessive processing of iso-α-acids under basic conditions results in the formation of humulinic acids. Humulinic acids do not demonstrate significant antibacterial, foaming or bitter properties, implying the importance of hydrophobicity for the elicitation of these properties.
From a brewing perspective then it is the iso- α -acids that are the principal bacteriostatic agents, due to their retention into the final product and their higher solubility in aqueous acidic media. However, in other food-related and fermentation settings, it is the beta-acids that are generally considered to be the most commercially important bacteriostatic species. For products for consumption this is undoubtedly influenced by the absence of bitterness of the beta-acids, whereas, for instance, in beer, bitterness of the iso- α -acids is detectable at around 10 – 15 μM (3 – 5 mg/l). The antibacterial applications of hop acids are diverse, and the examples from the recent patent literature reveals options for their use in:
- Foodstuffs (pastes, canned foods6)
- Fruit juices and other drinks7
- Sugar production8
- Antibacterial nappies and wet-wipes9
- Bioethanol production10
- Other processing media (e.g. in waters in paper manufacture11)
The successful application of naturally-occurring or easily accessible materials to enhance the quality of foods and beverages – both in production and in final product – has obvious attractions, not least of them being that the materials are proven to be safe to consume and, therefore not problematic if included in a potable product or in a material in contact with a food or drink product. Additionally, the range of hop resins and their derived products are relatively straight-forward to isolate in commercially viable quantities (for a review see Roberts and Wilson12). Thus, several hop product manufacturers routinely extract hop resins on a commercial scale using either liquid or supercritical CO2, achieving extracts containing levels of α -acids at 40 per cent (w/w) or higher and beta-acid levels around 15 per cent (w/w) or higher, depending on the variety extracted. A proportion of these extracts can then be isomerised in almost quantitative yield to the iso-alpha-acids, or processed to produce the chemically-modified iso- α -acid family (Figure 5). These latter are cited in several patents, although their unit cost is such that they would need to demonstrably outperform the cheaper (less-processed) variants to find widespread use in a specific application.
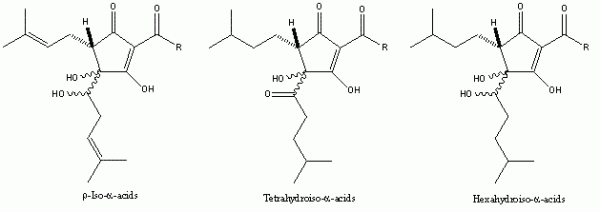

Figure 5 The chemically-modified iso-α-acids. Used by brewers because of their enhanced stability, they also exhibit bacteriostatic activity broadly in line with their hydrophobicity. Their cost and, for the tetrahydroiso- and hexahydroiso-α-acids, greater difficulty in achieving solubility in aqueous solution, are barriers to their widespread use.
Nonetheless, the hop acids cannot satisfy every need for microbial stability, as they are generally ineffective against Gram-negative bacteria and thus do not impact on the Enterobacteria (e.g. Salmonella, E. coli and the vibrio cholerae). The future development of the use of hop-derived compounds for enhancing food and drink quality through suppression of bacterial activity is likely to revolve around expanding current usages in the control of Gram-positive bacteria. It is interesting to speculate as to whether it is possible to resolve bitterness and antibacterial potency more fully, so that for potable products the required degree of potency can be achieved at levels below sensory detection. For the chemist there is also the tantalising possibility that one of a plethora of minor compounds found in resin extracts (many as yet unidentified; for some idea of possible scope here see Verzele and De Keukeleire13) could deliver some additional as yet unsuspected activity, providing a challenge to the breeder and the chemist alike to meet potential demand.
References
1. Snow, J., On the Mode of Communication of Cholera, 1855, Churchill, London
2. Hudson, J.R., Development of Brewing Analysis – A Historical Review, 1960, Institute of Brewing, London
3. www.barthhaasgroup.com/images/pdfs/ barthreport20092010_english.pdf (Accessed 13th March 2011)
4. Simpson, W.J., Journal of the Institute of Brewing, 1993, 99, 317-326
5. Simpson, W.J. and Smith, A.R.W., Journal of Applied Bacteriology, 1992, 72, 327-334.
6. Maye, J.P. and Beddie, D., Patent Application, 2000, Pub. no. WO 00/52212
7. Maca, H.W., Barney, M.C. and Ryder, D.S, Patent Application, 2006, Pub. no. WO 2006/041711
8. Maniscalco, F., Trevisan, G., Filippini, F. and Bertuzzi, S. Patent Application, 2009, Pub. no. EP 1837409
9. Barney, M.C., Navarro, A. and Ryder, D.S., Patent Application, 2002, Pub. no. 02/055119
10. Maye, J.P., Patent Application, 2009, Pub. no. US 2009/0042276
11. Breen, A.W., Davis, P.S., Mayer, M.J. and Singleton, F.L., Patent Application, 2003, Pub. no. US 6,547,971
12. Roberts, T.R. and R.J.H. Wilson, “Hops”, in Handbook of Brewing, edited by F. G. Priest and G. G. Stewart, 2006, CRC Press, Taylor & Francis Group, Boca Raton, Florida
13. Verzele, M. and De Keukeleire, D., The Chemistry and Analysis of Hop and Beer Bitter Acids, 1991, Elsevier, Amsterdam



