When are chocolates really finished?
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 6 November 2006 | Julia Strassburg, Nestle Research Center, Vers-chez-les-Blanc, Gottfried Ziegleder, Fraunhofer Institut Verfahrenstechnik und Verpackung and Steve Beckett, Nestle R&D Centre York | 1 comment
Unfinished crystallisation in freshly produced chocolates is one of the major reasons for fat bloom, especially for filled products. Chocolate shells, if insufficiently crystallised, show reduced resistance to oil-migration of fillings. The influence of two production parameters, cooling tunnel time and storage temperature, on the finished state of chocolates is investigated. It is found that the crystallisation in the chocolates is not finished when the products leave the cooling tunnel.
Many confectioners believe the production process of chocolate and confectionery products to be completed once a good temper has been achieved and a product with a shiny gloss and hard finish has been produced. However, this is not the case; many changes continue to occur during the post cooling tunnel time. Seven to twelve minutes in a controlled, low temperature are sufficient to give the majority of the cocoa butter an opportunity to crystallise, but significant liquid fat still remains. A common misconception is that keeping the chocolate at the lowest temperature possible accelerates the crystallisation of the remaining liquid cocoa butter. On the contrary, warmer temperatures in fact support crystallisation – cold temperatures actually decrease diffusion. Diffusion is the basic principle of transport for liquid cocoa butter throughout the already partially solidified chocolate. If diffusion is not possible, the molecules align themselves rather than move. Therefore, just as much attention must be paid to the confectionery after the tunnel as before (Seguine, 1995).
Unfinished crystallisation in freshly produced chocolates is one of the major reasons for fat bloom, especially for filled products. Chocolate shells, if insufficiently crystallised, show reduced resistance to oil-migration of fillings. The influence of two production parameters, cooling tunnel time and storage temperature, on the finished state of chocolates is investigated. It is found that the crystallisation in the chocolates is not finished when the products leave the cooling tunnel. Many confectioners believe the production process of chocolate and confectionery products to be completed once a good temper has been achieved and a product with a shiny gloss and hard finish has been produced. However, this is not the case; many changes continue to occur during the post cooling tunnel time. Seven to twelve minutes in a controlled, low temperature are sufficient to give the majority of the cocoa butter an opportunity to crystallise, but significant liquid fat still remains. A common misconception is that keeping the chocolate at the lowest temperature possible accelerates the crystallisation of the remaining liquid cocoa butter. On the contrary, warmer temperatures in fact support crystallisation – cold temperatures actually decrease diffusion. Diffusion is the basic principle of transport for liquid cocoa butter throughout the already partially solidified chocolate. If diffusion is not possible, the molecules align themselves rather than move. Therefore, just as much attention must be paid to the confectionery after the tunnel as before (Seguine, 1995).
Unfinished crystallisation in freshly produced chocolates is one of the major reasons for fat bloom, especially for filled products. Chocolate shells, if insufficiently crystallised, show reduced resistance to oil-migration of fillings. The influence of two production parameters, cooling tunnel time and storage temperature, on the finished state of chocolates is investigated. It is found that the crystallisation in the chocolates is not finished when the products leave the cooling tunnel.
Many confectioners believe the production process of chocolate and confectionery products to be completed once a good temper has been achieved and a product with a shiny gloss and hard finish has been produced. However, this is not the case; many changes continue to occur during the post cooling tunnel time. Seven to twelve minutes in a controlled, low temperature are sufficient to give the majority of the cocoa butter an opportunity to crystallise, but significant liquid fat still remains. A common misconception is that keeping the chocolate at the lowest temperature possible accelerates the crystallisation of the remaining liquid cocoa butter. On the contrary, warmer temperatures in fact support crystallisation – cold temperatures actually decrease diffusion. Diffusion is the basic principle of transport for liquid cocoa butter throughout the already partially solidified chocolate. If diffusion is not possible, the molecules align themselves rather than move. Therefore, just as much attention must be paid to the confectionery after the tunnel as before (Seguine, 1995).
Due to economic reasons the confectionery industry went through a change in recent years. Production times, and therefore also the cooling times, were shortened and the cooling temperatures lowered. As a consequence of these changes a well-known problem – fat bloom – has become increasingly relevant. This whitish haze or greyish layer that can appear on chocolate is critical because the consumer rejects it. Clusters of large fat crystals (5 μm or larger), which rise from the surface of the chocolates, cause scattering of light, which is perceived as fat bloom.
There are different mechanisms of bloom development, the most important of which is center bloom as a consequence of migration of filling oils to the shell – although the polymorphic transformation (change of crystals from the desired β (V) to the β (VI) modification) is by far more discussed. The very stable β (VI) crystals form needles, which penetrate the dark surface of the chocolate and are visible as a white layer (Hartel, 1999).
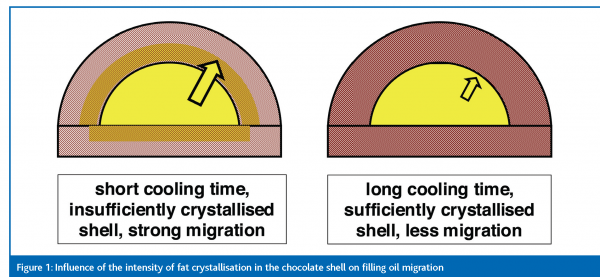
Liquid filling fats may migrate through the chocolate shell and transport or press triglycerides onto the surface where, as a consequence, they generate bloom. If the shell of a filled chocolate product is not sufficiently crystallised, it shows little resistance to bloom development. Fat migration from the center begins immediately after production and is more intense if the fat phase in the chocolate shell is in an unfinished state (Ziegleder, 1997). Furthermore, once intense migration has started and liquid triglycerides from the filling have invaded the chocolate shell, further crystallisation and stabilisation of the shell is hindered (figure 1). Hence, sufficient cooling time and optimum cooling conditions are essential for all types of filled chocolates and confectionery.
The awareness of this requirement begs the question: How finished are chocolates after production under varying conditions?
In our trials, crystallisation of a chocolate model system is studied. The setup is a pilot plant scale with 12 kg capacity. Plain chocolate bars of 103 mm length are produced. The chocolate mass used is a dark chocolate containing 33.7 per cent cocoa butter and 2 per cent anhydrous milk fat (AMF). The cocoa butter is of West African origin. Tempered chocolate is molded at 30 ºC before cooling. The molds are made from polycarbonate material.
Several production parameters are investigated
Tempering degree:
The chocolate is tempered in an AMK 10 three stage temperer to fulfill three different requirements: over tempered, well tempered and under tempered. The tempering degree is evaluated through use of a tempermeter.
Cooling tunnel time:
The chocolate is cooled in a tunnel of 12m length at constant air temperature of 15ºC for 7.5, 9 or 18 min. This temperature is considered to be the optimum temperature with respect to data from the literature: best results at 14-16ºC (Ziegleder, 1995) or at 13-15ºC (Weyland, 1998).
Storage temperature/time:
The finished chocolates are stored at 12ºC and 23ºC. Measurements (see below) are taken directly after demoulding, then 1 hour, 2 hours, 1 day, 2 days and 10 days after production. Some samples are placed into a laser extension rig at 12ºC and 23ºC, respectively.
Measurement methods
Solid Fat Content (SFC)
The solid fat content of the chocolate is measured by pulsed nuclear magnetic resonance (pNMR) with a Bruker Minispek according to Ziegleder et al. (1998), i.e. two measurements have to be carried out. First the chocolate is chopped up, placed into a glass tube and then measured. Second, the same chocolate is completely melted at 80ºC before it is measured again. The solid particles found in the second measurement are all non-fat; hence the true fat content can be mathematically determined.
Melting properties
This can be measured via differential scanning calorimetry (DSC) with a Perkin Elmer DSC 7: a chocolate sample of 2 – 10mg and a reference are heated from 15ºC to 40ºC at a rate of 5ºC/min. Afterwards the onset and endset temperature of the melting peak and the melting enthalpy are investigated.
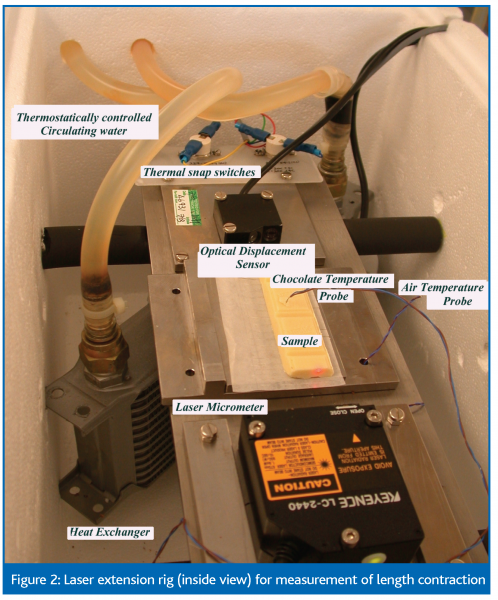
Length contraction
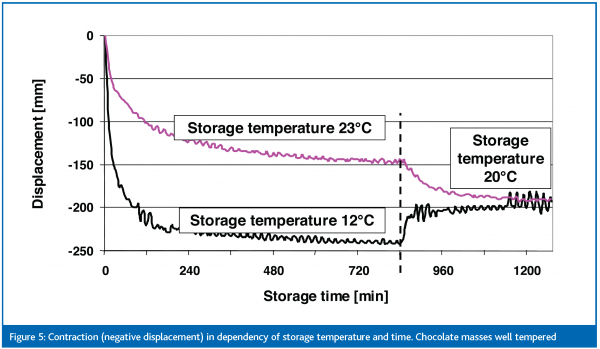
Length contraction is measured by means of a laser extension rig, which records the size of the chocolate sample over time through application of a laser beam on both sides of the sample, (figure 2).
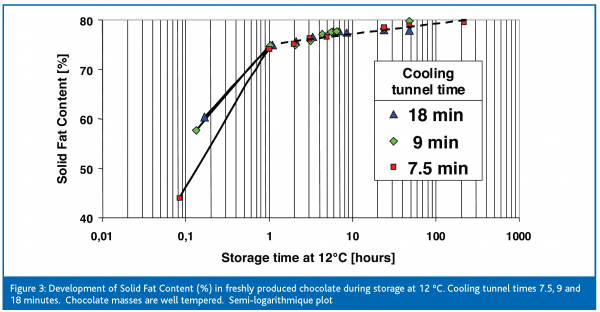
All trials show that chocolates leave the cooling tunnel in an unfinished and unstable state as can be seen when considering the solid fat content. Chocolates have a low solid fat content, i.e. between 45 and 60 %, approximately five minutes after demoulding (figures 3 and 4). The longer the residence time in the cooling tunnel is, the higher is the resulting solid fat content when measured directly after production. After 18 minutes of cooling between 55 and 60 % of cocoa butter crystallises, whereas for 7.5 minutes of cooling only 44 % becomes solid. Astonishingly the variation in the degree of temper has no convincing effect on the SFC.
Chocolate shells are commonly filled immediately after their passage through the cooling tunnel. After a short cooling tunnel time of, for example, 7.5 min these shells remain unfinished and are very susceptible to migration. Therefore, in many cases they will very quickly develop centre bloom.
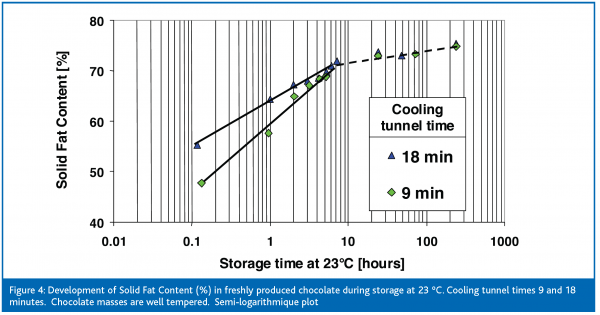
As expected, the solid fat content increases during the subsequent storage time. A storage temperature of 12ºC brings a rapid increase in the SFC to 76 % during the first hour of storage. Afterwards the crystallisation speed slows down, and a solid fat content of approximately 80 % may be reached after long-term storage. This is the maximum crystallisation that can be obtained, because the residual 20 % of the cocoa butter triglycerides are liquid at 12ºC. At a very high storage temperature of 23ºC the solid fat content increases more slowly and finally reaches 75 %. The solidification continues even at such high ‘summer-like’ temperatures. It illustrates the high reactivity of fresh products despite the previous cooling at 15ºC. The difference of 5 % of solid fat between storage at 23ºC and 12ºC were found in several trials. At 23ºC as well as 12ºC cocoa butter has reached its maximum crystalline level. This is 5 % higher at 12ºC than at 23ºC. At 23ºC chocolates are ‘softer’ since an additional 5 % of cocoa butter triglycerides remain molten. Therefore, the storage temperature has an immense influence on the migration resistance of chocolates. Migration of filling oils is much more intense at 23ºC. As shown, the diffusion coefficient for filling oils in chocolate increases by a factor of ten when raising the storage temperature from 12ºC to 23ºC (Ziegleder et al., 1996). Filled chocolates are usually stored between 15 and 18ºC. Conversely, plain chocolates could be given the chance to further crystallise when stored at 23ºC for a limited time. This can be seen when the melting properties are investigated.
Chocolates stored at 23ºC show a melting enthalpy (41,69§0.34 J/g), which is not significantly lower than the enthalpy for the samples stored at 12ºC (43,02 +/- 0.78 J/g, based on a p-value of 0.02 from ANOVA analysis it can be concluded that the groups of samples are not different). At first sight one could assume that the small difference between the values is due to the 5 % of cocoa butter that is liquid at this temperature but solid at 12ºC. The AMF is liquid at 23°C, and makes up for part of the lower enthalpy. However, this difference is smaller than the 5 % difference found for the solid fat content by pNMR. Hence, the difference is not only due to the quantity of crystals present.
The samples stored at 12ºC contain a significant amount of less stable crystals with lower melting enthalpy, which are not present at 23ºC. In contrast, the samples stored at 23ºC might contain fewer crystals but these dispose of a higher melting enthalpy, i.e. more stable forms. This assumption is confirmed by investigation of the onsets of the DSC melting curves. For chocolates stored at 12ºC the onset is more than 2ºC lower (24.6-25.1ºC versus 26.7-27.5ºC) than for the equivalent samples stored at 23ºC. The endsets remain similar. In general, chocolates stored for ten days at 23ºC show a slim DSC curve, as expected for cocoa butter crystals in β (V) form, whereas chocolates taken from 12ºC storage dispose of wide DSC melting curve reaching into the lower melting range.
Finally, the contraction of chocolates is measured using a laser. In most cases an absolute reduction in length in the range of 200 μm is found. This corresponds to a relative reduction of approximately 0.2 %. This result is not easy to interpret. The contraction due to fat crystallisation is competing with the thermal contraction. Figure 5 shows the displacement during the laser measurement for well tempered chocolate for 12 and 23ºC storage temperature. The displacement at 12ºC is more pronounced due to a greater temperature decrease (thermal contraction) and due to approximately 5 % more crystals. The samples need approximately four hours to reach the final displacement, with the strongest contraction being within the first hour. In general, the course of length contraction seems to correlate to crystallisation and solid fat increase. After 14 hours both chocolates are brought to 20ºC, where they reached the same final contraction. With respect to crystallisation, the volume contraction of chocolate shells is more intense than that of fillings. Hence, this could force oil migration because the filling oils are pressed into the tiny pores of the chocolate structure.
Summary
The state and development of freshly produced chocolates immediately after the cooling tunnel and up to several days after production is studied. The solid fat content of the chocolate is measured by means of pulsed NMR, the melting behavior by DSC and the contraction via length control with the help of a laser extension rig. It becomes evident that freshly produced chocolates are not finished once they leave the cooling tunnel. Further fat crystallisation and stabilisation occurs during the hours following production. The intensity of these processes depends on the storage temperature. This result is of special importance for filled chocolates. The susceptibility for centre migration is strongly dependent on the solid fat content of the freshly produced chocolate shells. Hence, even if the backing off (closing of the shell) issue remains, ideally the shells should not be filled before they have finished crystallisation with or without temperature support.





References
Beckett, S. (2000). The Science of Chocolate. Royal Society of Chemistry, London.
Hartel, R. (1999). Chocolate: fat bloom during storage. The Manufacturing
Confectioner, 89-99
Hartel, R., Martini, S., & Herrera, M. (2001). Effect of cooling rate on nucleation behavior of milk-fat-sunflower oil blends. J. Agric. Chem., 49, 3223-3229.
Lohmann, M. & Hartel, R. (1994. Effect of milk fat fractions on fat bloom in dark chocolate. JAOCS, 71(3), 267-277.
Seguine, E. (1995). It ain’t over until…The Manufacturing Confectioner, 55-58 Weyland, M. (1998). Shelf life of chocolate and compound coatings. The Manufacturing Confectioner, 121-140
Ziegleder, G. (1995). Kristallisation von Schokoladenmassen Teil IV: Kristallisation im Kühler. ZSW, Munich, 62-66.
Ziegleder, G. (1997) Fat migration and bloom. The Manufacturing Confectioner, 2, 43-44.
Ziegleder, G. (2000). Different causes of fat blooming. ZDS Fat Bloom Symposium, Solingen.
Ziegleder, G., Moser, C., & Geier-Greguska, J. (1996). Kinetik der Fettmigration in Schokoladenprodukten. Fett/Lipid (English translations www.britanniafood.com), 98, part I: 196-199, part 2: 253-256.
Ziegleder, G., Schwingshandl, I., & Brulheide, M. (1998). Beurteilung gelagerter Schokoladen anhand NMR-Kernspinresonanz. Süsswaren, 7/8, 330-334





An excellent article and highly educative. This really throws light on what happens to chocolate on its onward journey. Very Fortunate to have come across and read this.
Thanks
Best Regards.