Rapid and automated screening of priority β-agonists in urine using high resolution LC-MS technology
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 6 September 2011 | Thorsten Bernsmann and Peter Fürst, Chemical and Veterinary Analytical Institute, and Michal Godula, Thermo Fisher Scientific | No comments yet
Beta-agonists (β-agonists) are synthetically produced compounds that are widely known for their bronchodilatory and tocolytic effects. At higher doses, these substances also have anabolic effects and can promote live weight gain in food producing animals. Clenbuterol is the most commonly used β-agonist for growth-promoting purposes, despite the fact that there have been documented cases when consumption of liver and meat from animals treated with clenbuterol has resulted in serious human intoxication1. Increased levels of β-agonists and their analogs have been associated with acute toxic effects such as heart palpitation, muscle tremor, tetany and severe migraines.
Due to their adverse effects, the use of β-agonists has been banned by the European Union and other regulatory agencies worldwide. Nevertheless, monitoring programmes have shown that β-agonists are still illegally used by food producers and moreover, newly developed analogs with modified structures are being continuously introduced in routine practice. As a result, there is a clear need for quick and simple screening methods to routinely and accurately monitor levels of β-agonists in samples of animal origin, including urine, plasma and tissues.
The Chemical and Veterinary Analytical Institute Münsterland-Emscher-Lippe (CVUAMEL) is the central official laboratory for food and feed control in the Münster district of Germany. The Institute’s main purpose is to analyse food and feed samples in order to ensure public health in line with EU legislative requirements. To achieve this goal, CVUA-MEL also engages in the development of new liquid chromatography analytical methodologies. This article will discuss how the combination of high resolution Orbitrap mass spectrometry (Exactive LC-MS) and TurboFlow technology has enabled CVUA-MEL to achieve efficient, rapid and automated screening of urine samples taken from farms in order to detect whether priority β-agonists have been administered to animals.
Regulatory overview
Council Directive 96/22/EC2 prohibits the use of β-agonists and other substances that induce a hormonal or thyrostatic action in stockfarming. The Directive covers substances that have an anabolising action such as β-agonists and are used illegally in livestock rearing in order to stimulate the growth and yield of animals. According to the legislation, administering medicinal products based on β-agonists to animals may be authorised only for well-defined therapeutic purposes. Meat or products derived from animals to which β-agonists have been administered in accordance with the dispensatory provisions of this Directive may not be placed on the market for human consumption, unless the animals in question have been treated with legal veterinary medicinal products and the withdrawal period stipulated was observed before the animals were slaughtered. Additionally, the Directive identifies the need for maximum withdrawal periods to be set for veterinary medicinal products containing hormonal substances or β-agonists.
The Council Directive 96/23/EC3 requirement to monitor certain substances and residues in live animals and animal products specifies that EU Member States should draft a national residue monitoring plan for substances having anabolic effect, unauthorised substances, veterinary drugs and contaminants, including β-agonists. The Directive establishes the frequencies and level of sampling and the groups of substances to be controlled for each food commodity.
Commission Decision 2002/657/EC4 implements Council Directive 96/23/EC and details criteria and procedures to be used for the validation of analytical methods to ensure the quality and comparability of analytical results generated by official laboratories. In addition, the Decision establishes common criteria for the interpretation of test results and introduces a procedure to progressively enforce minimum required performance limits (MRPL) for analytical methods employed to detect substances for which no permitted limit has been established. This is particularly important for substances not authorised for use or specifically prohibited in the EU, such as β-agonists.
To ensure regulatory compliance and safeguard consumer health, a stipulated number of samples must be taken and analysed in order to detect illegal drugs administered in animals and to ensure that withdrawal periods are being upheld by farmers.
Conventional technologies
Molecularly imprinted polymer (MIP) solid phase extraction (SPE) column clean-up has been traditionally used for sample preparation during determination of β-agonists in samples of animal origin. However, this technique is associated with a number of shortcomings that limit its efficiency. MIP synthesis is difficult to control due to uncertainties relating to polymer architecture, chemical environment diversity and site specificity. This is a particularly crucial limitation given that different MIPs must be synthesised for each new analyte or class of analytes. Factors such as the gel effect during cross linking contribute to the heterogeneity of imprinted matrices, whereas the restricted mobilities and high local viscosities noted in the latter stages of MIP synthesis can lead to residual reactive groups in the final material.
One of the detection techniques being used at CVUA-MEL is ultra performance liquid chromatography (UPLC) coupled with Time-of- Flight (TOF) MS. The major drawback of this technique is the occurrence of matrix effects, which can reduce or enhance substantially the response signal, thereby delivering unreliable results. Additionally, processing and reviewing TOF screening data can be a complex and time-consuming task, requiring positive peaks to be first identified and then quantified. A further shortcoming of the technique is that the transfer from qualitative to quantitative processes is often performed manually, thereby introducing a higher probability of errors and placing a greater burden on analysts.
A new method has been introduced, combining high resolution Orbitrap LC-MS technology and TurboFlow technology, offering significant gains in simplicity, speed and sensitivity for high-throughput food safety laboratories (see Figure 1).

Figure 1 A comparison of previous CVUA β-agonists methods workflow (left) with Aria TLX/Exactive automated clean-up approach
Materials and methods
A rapid and automated method for screening of priority β-agonists in urine samples was developed by CVUA-MEL. Liquid chromatography mass spectrometry analyses were performed using the benchtop Orbitrap single stage Exactive LC-MS (Thermo Fisher Scientific, Bremen, Germany). The LC-MS instrument was coupled to a TurboFlow system Aria TLX-1 consisting of loading and eluting pumps, a switching valve module and an autosampler (Thermo Fisher Scientific, Franklin, USA). All LC-MS measurements were performed at resolving power settings of 50,000 FWHM, while separation of analytes was carried out on a Hypersil GOLD Phenyl column 4,6 x 100 mm x 5 μm (Thermo Fisher Scientific, Runcorn, UK).
The optimisation of the TurboFlow method was performed using a standard solution containing salbutamol, clenbuterol and mabuterol at 100 μg.L-1 levels. These compounds were selected for optimisation due to their physico-chemical properties and strong dependency on the pH of the mobile phases. During method development, specific TurboFlow parameters were optimised, including column selection whereby evaluation of different types of columns was undertaken with respect to the retention of analytes. The composition and pH of the loading mobile phase as well as the pH, flow rate and time of the elution mobile phase were also optimised. Additionally, it was possible to optimise the initial gradient composition to effectively trap and focus all compounds of interest.
The optimised TLX method was then applied to the analysis of spiked and unspiked urine samples and standard solutions. The urine was spiked at different concentration levels, centrifuged for 10 minutes and diluted with water 1:1; pH was adjusted to six with 0.1M HCl. Prepared urine samples were directly injected into the TLX/Exactive system. Calibration was performed using urine matrix matched standards at concentration levels of 0.25; 0.5; 1; 5; 10; 15; 20 and 25 μg/L. The typical chromatogram of the standard solution at 0.25 μg/L and spiked urine sample at concentration level of 1 μg.L-1 is shown in Figure 2 and Figure 3.
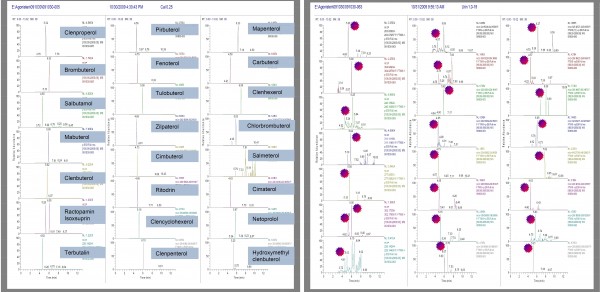

Figure 2 Extracted ion chromatograms of beta-agonists at 0.25 μg/L concentration level. Figure 3 Extracted ion chromatograms of beta-agonists at 1 μg/L concentration level in urine
Results
Table 1 shows the group of priority beta-agonists analysed with this method. With the use of this method, all analysed compounds could be successfully detected at low μg.L -1 concentration levels in urine. In neat standard, all compounds are detected easily at 0.25 μg/L. In addition, in a few cases, coelutions occurred (salbutamol, terbutalin, pirbuterol) but the peak could be distinguished from the matrix.
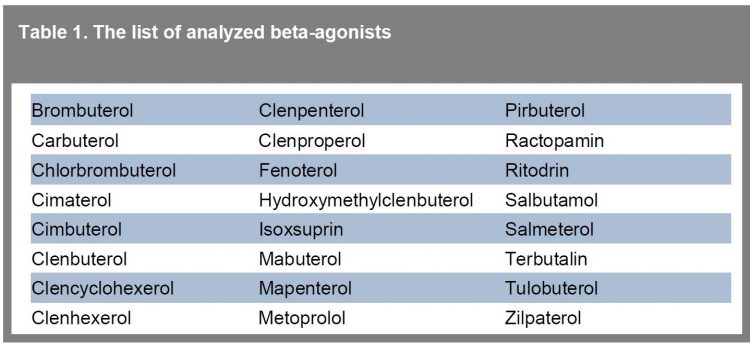

Table 1 The list of analyzed beta-agonists
Table 2 summarises detailed performance characteristics of the method evaluated by analyses of urine samples spiked at 1 μg.L -1. For estimation of precision and accuracy, the spiked samples were analysed in six replicates. Carryover sample (blank urine) was analysed immediately after the spiked samples. All calibrations were carried out using matrix matched standard solutions to eliminate matrix effects during the ionisation step.
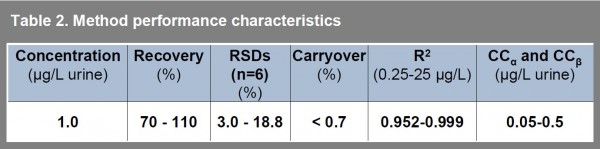

Table 2 Method performance characteristics
Conclusion
A rapid, sensitive and automated method has been developed for screening urine samples taken from farms in order to detect whether β-agonists have been administered to animals. Combining high-resolution Orbitrap LC-MS technology and TurboFlow clean-up technology has enabled considerable improvement of laboratory throughput and minimisation of manual work. The method provides a unique tool for quick and simplified urine sample screening for residues of β-agonists in compliance with current stringent legislation.
References
- Botsoglou, N.A., Fletouris D.J., Drug Residues in Food. Pharmacology, Food Safety and Analysis, Marcel Dekker: New York, 2001
- COUNCIL DIRECTIVE 96/22/EC of 29 April 1996, concerning the prohibition on the use in stockfarming of certain substances having a hormonal or thyrostatic action and of beta-agonists, and repealing Directives 81/602/EEC, 88/146/EEC and 88/299/EEC, http://eurlex. europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG: 1996L0022:20081218:EN:PDF
- COUNCIL DIRECTIVE 96/23/EC of 29 April 1996, on measures to monitor certain substances and residues thereof in live animals and animal products and repealing Directives81/602/EEC, 85/358/EEC and 86/469/EEC and Decisions 89/187/EEC and 91/664/EEC,http://ec.europa.eu/food/food/chemical safety/residues/council_directive_96_23ec.pdf
- COMMISSION DECISION (2002/657/EC) of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results (notified under document number C(2002) 3044), http://eurlex. europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2002: 221:0008:0036:EN:PDF
About the authors
Dr. Thorsten Bernsmann is a food chemist. He studied at the University of Münster and received his PhD in 2003. From December 2002, he has been at the Chemical and Veterinary Analytical Institute in Münster as Laboratory Head ‘LC-MS, GC-MS’. His main working areas are the determination of organic contaminants in food and feed, trace analysis with GC-MS, GC-MS/MS, LCMS/ MS and LC-MS/Orbitrap and analysis of marine biotoxins in shellfish.
Prof. Dr. Peter Fürst is a food chemist. He studied at the University of Münster and received his PhD in 1982. Since 1981, he has been at the Chemical and Veterinary Analytical Institute in Münster as Head of the department ‘Central Analytical Services’. His main working areas are the determination of dioxins, PCBs and brominated flame retardants in food and feed and analysis of pharmaceutically active compounds with special emphasis on illegal drugs. He is an Honorary Professor at the Faculty of Chemistry at the University of Münster and a member of the panel on Contaminants in the Food Chain of the European Food Safety Authority (EFSA).
Michal Godula, PhD is a European food safety specialist of Thermo Fisher Scientific Food Safety Group. He has a wide experience in many areas of the analysis of organic and inorganic contaminants in food with the focus in mass spectrometry. He is responsible for technical and application support of key customers and managing of Thermo’s scientific collaboration projects within EU.






