Freeze-drying in the coffee industry
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 23 April 2015 | Davide Fissore | 3 comments
Freeze-drying is a process used in food processing to remove water from foodstuffs, with the goal of increasing their shelf life. The process consists of various steps: At first product temperature is lowered, usually to about -40°C, thus causing freezing of the free water. Later, the pressure in the equipment is lowered and sublimation of the frozen water occurs (primary drying). Finally, the bound water is removed from the product, usually increasing product temperature and further decreasing the pressure in the equipment, thus reaching the target value of residual moisture (secondary drying).


When processing foodstuffs the very low operating temperatures and the gentle drying conditions of a freeze-drying process avoid aroma and colour deterioration, as well as nutrient degradation, thus making this process particularly suitable for obtaining high quality products.
Freeze-drying is a key stage in instant coffee production. Coffee beans are first roasted and ground, then dissolved into hot water. By this process coffee flavour, aroma and colour are extracted from the coffee grounds, and a highly concentrated liquor is obtained (generally the coffee solution is about 15–30% coffee by mass at the end of this extraction process).
After filtration, the coffee extract is dried to get the solid soluble coffee. The liquor is frozen to about -40°C to form a thin layer that is then broken into tiny pieces. These granules are then loaded into the freeze-dryer: both batch and continuous plants are used to freeze-dry the frozen product. A batch process is used for low capacities (generally ranging from 50-7,000kg of powder per day), while a continuous process is used for large capacities (generally ranging from 7,000-25,000kg of powder per day).
A batch plant consists of a cabinet with a door for product loading/unloading. In the cabinet there are various shelves: hot liquid is circulated through the system in such a way that the heat required for ice sublimation is properly transferred to the product. The frozen product can be directly loaded onto the shelves, or it can be placed onto a wagon hanging on trolleys and placed in the cabinet in such a way that product trays are positioned between the heating shelves: in this case the product is heated only by radiation from the shelves.
In case of a continuous freeze-dryer the cabinet is a long cylindrical chamber: the trays containing the product enter through an airlock system that avoids breaking the vacuum, and they are moved along the cabinet. Also in this case heat is transferred to the product using heating shelves. The product can be directly loaded onto these shelves, or it can be placed between the heating shelves as in batch plants. Both batch and continuous plants comprise a vacuum pump, a condenser for the water vapour, and a de-icing unit to melt the ice accumulated in the condenser (thus maintaining high condensing efficiency).
Process design and optimisation
With the goal of retaining all the desired characteristics (e.g. colour, appearance, shape, texture and taste) in the final product the freeze-drying process has to be properly designed, i.e. the operating conditions (the pressure in the drying chamber and the temperature of the heating fluid) have to be correctly selected. Generally, the target is to maintain product temperature below a limit value that is a characteristic of the product being processed. In doing so it is also possible to get a high specific surface area in the final product, allowing for fast and easy rehydration. Another relevant concern is the duration of the process, and the related energy requirement, which is higher with respect to that of other drying processes: about 2.5kWh are required to remove 1kg of water in a vacuum freeze-drying process, as reported by Claussen et al.1.
An extensive experimental investigation, based on a trial and error approach, is usually carried out to identify the ‘optimal’ operating conditions that allow for obtaining a product with the desired characteristics. Taking into account the Guidance for Industry PAT issued by the US Food and Drug Administration in 2004 a different approach should be used to design the process: product quality has to be built into the process, or it should be by design, and no longer tested at the end of the process in the final product.
In this framework the use of mathematical modeling appears to be particularly promising: in fact, a mathematical model allows simulating in silico the evolution of the process for the selected values of the operating conditions, thus determining drying duration and product temperature without carrying out a ‘real’ cycle. Few experiments are in any case required to get the values of model parameters, but the duration of the cycle development stage is significantly reduced and, at the end of the investigation, a deep understanding of the effects of the operating conditions on product dynamics is obtained. Results are expressed by means of a diagram where the values of the operating conditions that allow obtaining a product with the desired characteristics, i.e. the design space, are put in evidence2.
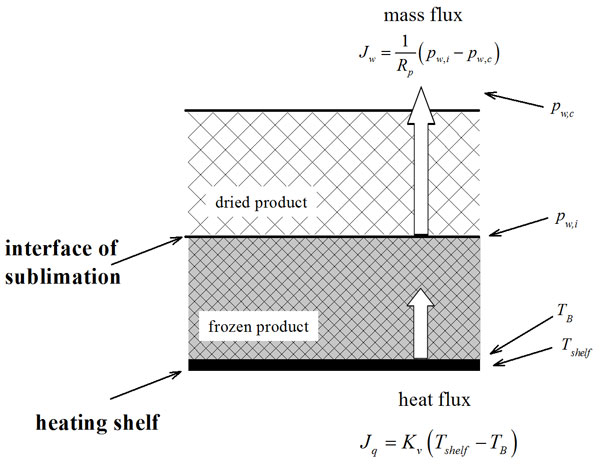

Figure 1
When using this approach, the selection of a ‘suitable’ model of the process is (evidently) of outmost importance. The selection of the model is influenced by existing knowledge about the system, by the data available, and by the objective of the study. Besides, the model contribution in assuring the quality of the product has to be taken into account. The ICH-Endorsed Guide for ICH Q8/Q9/Q10 Implementation issued in 2011 distinguishes among low, medium and high impact models. Low impact models are typically used to support product and/or process development, while medium impact models are used in assuring the quality of the product (but they are not the sole indicators of product quality). The prediction of high impact models is a significant indicator of the quality of the product. Obviously, the accuracy of the model has to increase, moving from low and medium to high impact models.
Focusing on a simple model used for process design, the heat flux from the heating shelf to the product can be described by the following equation3,4:
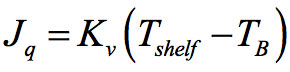
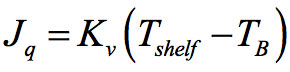
Where Kv is a heat transfer coefficient that accounts for the various mechanisms of heat transfer to the product, TB is the temperature of the product at the bottom of the container used and Tshelf is the temperature of the heating shelf (see Figure 1). The water vapour flux from the sublimation interface to the chamber can be described by the following equation:
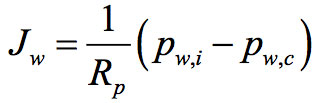
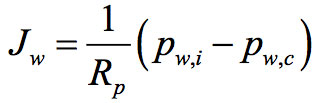
Where Rp is the resistance of the dried product to vapour flux, and pw,i and pw,c are, respectively, the water vapour partial pressure at the sublimation interface and in the drying chamber (see Figure 1). Various techniques are available to estimate Kv, and they were reviewed by Pisano et al.5. With respect to Rp, the most common technique used to estimate this parameter is the pressure rise test (see, among the others, Fissore et al. (2011)6, for details).
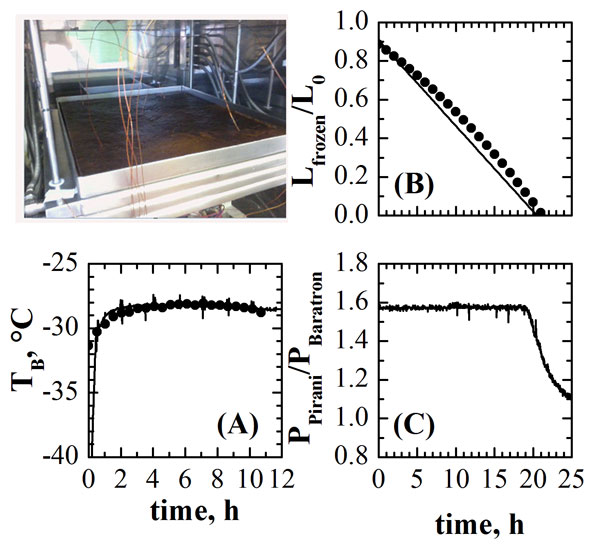

Figure 2
Once model parameters are known, the dynamics of the process can be described by the simplified one dimensional model of Velardi and Barresi4: it is composed by the energy balance for the frozen product and the mass balance for the dried layer. Figure 2 shows an example of the results that can be obtained when simulating the freeze-drying of a coffee extract (25% by weight of coffee) processed in tray, with a thickness of frozen product of 12mm (Tshelf = -5°C, Pchamber = 5Pa). Graph A shows the values of product temperature measured by thermocouples (symbols) inserted in the frozen product (shown in the picture of the system) and the values calculated using the model (line). Graph B shows the values of frozen layer thickness estimated using the pressure rise test (symbols) and those calculated using the model (line).
In both cases a very good agreement is obtained between experimental measurements and process simulation, thus evidencing the adequacy of the mathematical model to describe the evolution of the product for the selected operating conditions. A further validation of the drying duration is shown in Graph C, where the ratio between the pressure measurements provided by capacitance (626A Baratron, MKS Instruments, Andover, MA, USA) and thermal conductivity (Pirani PSG-101-S, Inficon, Bad Ragaz, Switzerland) gauges is shown. This ratio remains almost constant throughout the primary drying stage and then decreases when the ice sublimation is completed7. A good agreement is obtained when this occurrence is compared to the conclusion of the ice sublimation as predicted by the mathematical model (Graph B).
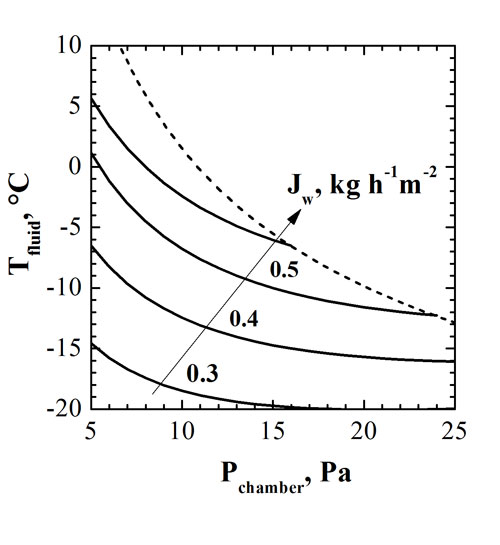

Figure 3
Once the model has been validated, it can be used to investigate the effect of the operating conditions (heating shelf temperature and chamber pressure) on the product temperature and on the sublimation flux. Figure 3 shows an example of the results that can be obtained by this way: the iso-flux lines are shown, pointing out that, in this case, higher values of sublimation flux are obtained working at high values of shelf temperature and low values of chamber pressure. The dashed line evidences the design space of the process – with the goal to preserve product quality (i.e. to maintain product temperature below the selected limit value, -25°C in this case, corresponding to the glass transition temperature of the dried product). For each selected value of chamber pressure it is necessary to keep the temperature of the heating shelf below the limit value identified by the dashed line in the figure. This investigation can be improved by considering the energy utilisation efficiency, as recently proposed by Fissore et al.8 using the instant coffee production as a case study.
References
- Claussen, IC, Ustad, TS, Strømmen, I, Walde, PM (2007). Atmospheric freeze drying – A review. Drying Technology 25: 957-967.
- Giordano, A, Barresi, AA, Fissore, D (2011). On the use of mathematical models to build the design space for the primary drying phase of a pharmaceutical lyophilization process. Journal of Pharmaceutical Sciences, 100: 311-324.
- Pikal, MJ (1985). Use of laboratory data in freeze-drying process design: heat and mass transfer coefficients and the computer simulation of freeze-drying. Journal of Parenteral Science and Technology, 39: 115-139.
- Velardi, SA, Barresi, AA (2008). Development of simplified models for the freeze-drying process and investigation of the optimal operating conditions. Chemical Engineering Research and Design, 87: 9-22.
- Pisano, R, Fissore, D, Barresi, AA (2011). Heat transfer in freeze-drying apparatus. In: dos Santos Bernardes, MA (Ed). Developments in Heat Transfer. Rijeka, InTech.
- Fissore, D, Pisano, R, Barresi, AA (2011). On the methods based on the Pressure Rise Test for monitoring a freeze-drying process. Drying Technology, 29: 73-90.
- Patel, SM, Doen, T, Pikal, MJ (2010). Determination of the end point of primary drying in freeze-drying process control. AAPS Pharmaceutical Science Technology, 11: 73-84.
- Fissore, D, Pisano, R, Barresi AA (2014). Applying Quality-by-Design to a coffee freeze-drying process. Journal of Food Engineering, 123: 179-187.
About the author


Davide Fissore is Associate Professor at Politecnico di Torino (Italy). His research activity is focused on process modeling and optimisation, and on the design and validation of model-based tools for process monitoring and control. One of the topics of this research is the freeze-drying of pharmaceuticals and foodstuffs. In particular, he has proposed various devices to monitor and optimise in-line (using a control system) or off-line (using the design space of the product) the freeze-drying process for a given product. Recent research projects have addressed the use of non-aqueous solvents for pharmaceutical freeze-drying, and the freeze-drying of suspensions containing nanoparticles.








The coffee powder mesh size is not mentioned. In nestle coffee the roasted beans are ground and extracted by passing the mass thru scieves and recycling till tss is achieved .After this the mass is concentrated to 65 tss in vacuum evaporator with aroma recovery . After that the coffee concentrate is spray dried and flavour added back .
In case of freeze drying the flavour is added back before freezing the concentrate .
This process reduces cost of energy during freeze drying.
And flavours are retained better in freeze drying then spray drying .
Please suggest
I have read whole article. Main question arises in my mind is how to maintain the shelf temperature at -5 degree C in the freeze dryer?
The article describes in very good detail the process of freeze-drying.
When describing this process as applied to coffee it is described in simple terms well and clear. This is a minor error in the choice of words in the following statements of the description:
“Freeze-drying is a key stage in instant coffee production. Coffee beans are first roasted and ground, then dissolved into hot water. By this process coffee flavour, aroma and colour are extracted from the coffee grounds, and a highly concentrated liquor is obtained (generally the coffee solution is about 15–30% coffee by mass at the end of this extraction process).”
Firstly, not all instant coffee is produced using the freeze-drying. Simple instant coffee powder is produced using a spraying of the liquid into a high heat to achieve the drying quickly resulting in a fine dry powder. Some manufacturers add an additional process to combine the powder into larger pieces called agglomerated. As described freeze-drying achieves the desired improvements. The process done with the coffee as: “Coffee beans are first roasted and ground, then dissolved into hot water”, has a mistake at the end. Ground coffee is not dissolved into hot water, since it will not dissolve. Rather coffee is brewed or percolated in hot water to extract from the coffee beans the flavour, colour, aroma and other constituents like caffeine, anti-oxidants, etc., all which extract into the hot water during this process.
Thank You for making this correction.