Benefit risk assessment of microbial food cultures
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 6 November 2012 | François Bourdichon, Nestlé Research Centre; Joerg Seifert, International Dairy Federation and Egon Bech Hansen, Technical University of Denmark | No comments yet
Fermentation as a chemical process was initially described in the mid-19th century by Louis Pasteur as ‘a vie sans l’air’, the metabolic process of deriving energy from organic compounds without the involvement of an exogenous oxidising agent. Fermentation, as a process for manufacturing fermented foods, is today used more broadly than the historical definition of fermentation. Fermented foods have been subjected to the action of microorganisms during which desirable biochemical changes occur, causing significant modification to the food matrix2,13.
Fermented foods are typically associated with local and traditional food consumption. The growing body of evidence with regard to microorganisms and their ecological role in the food matrix has led to industrial application of the process of fermentation starting in the early 20th century through use of specific dedicated microbiota with various levels of characterisation.
In recent decades, the use of microbial food cultures (MFC) has come under various regulatory frameworks in many countries, directly or indirectly. Several of these regulatory frameworks put emphasis on ‘the history of use’, ‘traditional food’, or ‘general recognition of safety’ without clear guidelines for the expected level of evidence.
Fermentation as a chemical process was initially described in the mid-19th century by Louis Pasteur as ‘a vie sans l’air’, the metabolic process of deriving energy from organic compounds without the involvement of an exogenous oxidising agent. Fermentation, as a process for manufacturing fermented foods, is today used more broadly than the historical definition of fermentation. Fermented foods have been subjected to the action of microorganisms during which desirable biochemical changes occur, causing significant modification to the food matrix2,13. Fermented foods are typically associated with local and traditional food consumption. The growing body of evidence with regard to microorganisms and their ecological role in the food matrix has led to industrial application of the process of fermentation starting in the early 20th century through use of specific dedicated microbiota with various levels of characterisation. In recent decades, the use of microbial food cultures (MFC) has come under various regulatory frameworks in many countries, directly or indirectly. Several of these regulatory frameworks put emphasis on ‘the history of use’, ‘traditional food’, or ‘general recognition of safety’ without clear guidelines for the expected level of evidence.
Fermentation as a chemical process was initially described in the mid-19th century by Louis Pasteur as ‘a vie sans l’air’, the metabolic process of deriving energy from organic compounds without the involvement of an exogenous oxidising agent. Fermentation, as a process for manufacturing fermented foods, is today used more broadly than the historical definition of fermentation. Fermented foods have been subjected to the action of microorganisms during which desirable biochemical changes occur, causing significant modification to the food matrix2,13.
Fermented foods are typically associated with local and traditional food consumption. The growing body of evidence with regard to microorganisms and their ecological role in the food matrix has led to industrial application of the process of fermentation starting in the early 20th century through use of specific dedicated microbiota with various levels of characterisation.
In recent decades, the use of microbial food cultures (MFC) has come under various regulatory frameworks in many countries, directly or indirectly. Several of these regulatory frameworks put emphasis on ‘the history of use’, ‘traditional food’, or ‘general recognition of safety’ without clear guidelines for the expected level of evidence.
Fermented foods generally have a very robust record of being safe. Application of the principles for the safety of fermented foods and good manufacturing practices is seen as a possible path for improving the overall quality and the nutritional value of the food3,10.
Fermentation plays an important role for food safety of certain food products. It has been understood as a positive action against undesired microorganisms through either ecological completion, bacteriostatic or bactericidal activity. The evaluation of ‘positive’ microorganisms ought to be conducted through a ‘classical’ microbiological risk assessment (MRA) as used for the ‘negative’ microorganisms – i.e. pathogenic species7.
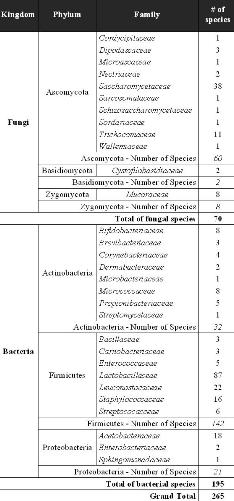
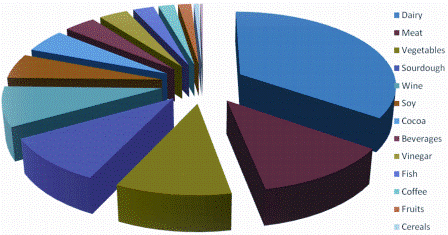
In 2002, the International Dairy Federation (IDF) and the European Food and Feed Cultures Association (EFFCA) developed an inventory of microorganisms with a documented history of use in food8,9. In the past 10 years, this inventory has become a reference for food cultures in practical use. In order to capture the growing body of scientific evidence and the evolution of taxonomy, a major update was established and published in 20121. The updated list of microorganisms (Table 1) is based on the latest taxonomic usage. The scope of the list has been widened to a larger range of food matrices (dairy, meat, fish, vegetables, cereals, beverages and vinegar) as well as the inclusion of traditional food products from all over the world (Figure 1). Although the length of the list has been more than doubled in this recent update, it was not possible to consider all types of fermented foods and microbial species, owing to the lack of scientific data required to demonstrate some still largely unknown food processes in terms of their biochemical mechanisms.
It is to be noted that the European Food Safety Authority (EFSA) Biohazard Panel has published an internal assessment of microorganisms intentionally added to the food chain6. The approach of EFSA differs from that of IDF/EFFCA with respect to species which are not necessarily intentionally added, but just naturally present. Metabolic pathways and the food matrix are also considered in the IDF approach, which is not the case in the qualified pre – sumption of safety (QPS) assessment as discussed during the EFSA QPS Scientific colloquium of autumn 2004. The list of species published and updated yearly by EFSA Biohazard Panel for its internal assessment of strains is therefore different from the list proposed by IDF/EFFCA. As the two approaches are based on a different rationale, comparison of these two lists should be made with caution.
History of use
The concept of ‘history of safe use’ has appeared recently in regulations and in safety assessment guidance. One definition of ‘history of safe use’ proposed by Health Canada states “significant human consumption of food over several generations and in a large, genetically diverse population for which there exist adequate toxicological and allergenicity data to provide reasonable certainty that no harm will result from consumption of the food”. A long history of apparently safe use of given microorganisms for the making of a given food suggests a very high safety level for the consumption of such products.
In order to evaluate the history of safe use of a microorganism it is necessary to document not just the occurrence of a microorganism in a fermented food, but to evaluate if the presence of the microorganism is indeed beneficial, or fortuitous, and not undesired and whenever possible document the ‘mechanism of action’ of one species in a food process.
Desired properties of MFCs in fermented foods
While fermentation is estimated to be used by man since the Neolithic period (around 10,000 years B.C.), the study of a scientific rationale behind fermentation was impossible until microorganisms had been discovered in 1665 by van Leeuwenhoek and Hooke. After this discovery, two more centuries elapsed before the era was initiated by Louis Pasteur.
The role of a sole microorganism, Bacterium lactis (Lactococcus lactis) in the process of curdling milk was only demonstrated a little more than a century ago in 1877 by Sir John Lister. Fermentation plays different roles in food processing such as:
- Preservation of food through formation of inhibitory metabolites such as organic acids, often in combination with decreased water activity (by drying or use of salt)
- Improving food safety through inhibition of pathogens or removal of toxic compounds (e.g. Cassava fermentation in Africa)
- Improving the nutritional value and organoleptic quality of the food.
Undesirable properties of MFCs
Recent discovery of rare events of adverse effects caused by microorganisms of species similar to those commonly found in fermented foods raised some concerns. There is a need to obtain more scientific evidence on this newly identified hazard, depending on either the food matrix or the susceptibility of the host.
The body of scientific documentation has developed significantly in the past 20 years. Based on the available scientific evidence, an IDF taskforce, including repre – sentation of EFFCA, has updated the initial publications8,9 to consider the following topics1.
Opportunistic infections
Commensal bacteria have been described to cause infections in patients with underlying disease. Owing to its natural presence in different sites of the human body and in fermented food products, the genus Lacto – bacillus has gained particular attention. Lactobacillus infections occur at a very low rate in the generally healthy population – estimated 0.5-1 million per year. Infections with the commonly used yeast and mould species are rare events as well. Most of the infections are due to opportunistic pathogens not recognised as MFC and affect immune-compromised patients and hospitalised patients.
Toxic metabolites and virulence factors
Biogenic amine formation in fermented foods by lactic acid bacteria (LAB) has recently been reviewed11. The presence of mycotoxin genes also raises safety concerns, although the level of expression within fermented food is very unlikely to cause any health hazard. Within fungi, the potential for antibiotic production is also an undesired property. A specific risk assessment should be conducted on strains presenting these undesirable properties, even if they belong to a species with a long history of use in food.
Antimicrobial resistance
The emergence and spread of antimicrobial resistance (AMR) is a major global health concern. The role of MFC in the spread of AMR has been assessed in fermented foods as well as more specifically for probiotic food products. Results of such studies confirm the role of a reservoir of AMR genes from the food microbiota, without identifying any major health concern to date.
Regulatory systems and legal terms
MFCs have not been defined legally. To fill this gap, EFFCA has proposed the following definition: ‘Microbial food cultures are live bacteria, yeasts or moulds used in food production’. MFC preparations are formulations, consisting of one or more microbial species and/or strains, including media components carried over from the fermentation and components which are necessary for their survival, storage, standard – isation, and to facilitate their application in the food production process. These preparations can be made using single strains, a mix of different strains and also an undefined culture where the composition of microorganisms is not strictly defined in terms of enumeration and identification of species present, e.g. kefir. Also, spontaneous fermentation without inocu – lation can be used for food fermentations as is the common practice for wine and some types of beer (lambic).
According to the European regulations, uncertainty may arise when a decision is required on whether or not a food associated microorganism has to be deemed as novel. Changes in taxonomy may complicate this decision as well as progress of isolation techniques with regard to the use of more sensitive methods. According to Vogel et al., considering generic properties of microbial food fermentation species (characterised strains), their history of safe use and their specific benefit risk assessment7, it is reasonable to assume that newly described or newly identified micro – organisms involved in the fermentation processes of traditional food should not necessarily be considered as novel14.
Taxonomy
Taxonomy and systematics constitute the basis for the regulatory frameworks for MFC. It is thus somewhat unfortunate that the definition of microbial species as a taxonomic unit lacks a theoretical consensual basis12.
Molecular methods have broadened scientific understanding of bacterial and fungal biodiversity. As a consequence, it has been necessary to adopt a pragmatic approach to defining species. Microbial taxonomy has changed considerably in recent decades. There is no reason to believe that microbial taxonomy has found its final form, switching from a phenotypic-based classification to a genetic-based one.
Advances in genetics and physiology have greatly increased our understanding of microbial phylogeny and have led to substantial changes in microbial taxonomy. These advances have generally facilitated the safe use of microorganisms, but have also led to some challenges for regulators, e.g. some newly described species with a long history of use may possibly be considered novel. The more difficult problem to reconcile within the regulatory framework is resolving the lack of consensus on the definition of the taxonomical unit or species.
In practice, this means that a species is represented by a type strain with strains showing a high degree of phenotypic and/or genotypic similarity to that type strain and regarded as belonging to the same species. Whilst objective measures of relatedness have been proposed (such as percentage of genome hybridisation or sequence similarity) there is still no simple definition, neither of the species level nor the strain level as a taxonomical unit
Food for thought
Either in traditional fermented foods or as new opportunities, the rational use of micro – organisms in our diet opens new perspectives. Furthermore, following the discovery of penicillin by Fleming in 1928, the use of micro – organisms in fields other than the food industry has been investigated extensively by the pharmaceutical industry and others sectors. Lactococcus spp. is used for its potential role in vaccination, microorganisms are also used for the specific production of biogenic compounds (e.g. natamycin from Streptomyces natalensis).
Microbial strains in food products are sometimes also used beyond fermentation for improved health effects on the host. These have been defined by a joint working group of FAO/WHO4,5 as probiotics, ‘Live microorganisms which when administered in adequate amounts confer a health benefit on the host’. For probiotics, numerous guidelines for safety and efficacy demonstration are proposed and discussed in the existing scientific literature.
Microbiological research mostly focuses on the pathogenicity of certain microorganisms while the positive role of some microorganisms for improving technological and/or nutritional properties of food has largely been neglected. While there has been a recent focus on the emerging science of the preponderant role of our own microbiota, our ‘other genome’, from the skin, gut, and other mucosa, research activities remain to be carried out on characterising traditional fermented foods consumed for centuries.
Updating an inventory of microbial species used in fermented food products is a neverending task. Critical influences are the evolving taxonomy, the identification of new micro – organisms, and new descriptions of roles of microorganisms in fermented foods. The exclusion of a microbial species from an established inventory shall be considered in cases of new evidence of potential risks, e.g. opportunistic infections or toxin production. It has to be borne in mind that whole food cannot be tested according to the standard safety evaluation principles used for single substances such as pharmaceuticals, food additives or food contaminants.
IDF has therefore dedicated a specific expert group to work on a continuous update of the recently published up-to-date inventory based on thorough evaluation of new scientific evidence to be provided. IDF invites submissions for addition of new species or withdrawal of species currently contained in the inventory.
References
1. Bourdichon, F., Casaregola, S., Farrokh, C., Frisvad, J.C., Gerds, M.L., Hammes, W.P., Harnett, J., Huys, G., Laulund, S., Ouwehand, A., Powell, I.B., Prajapati, J.B., Seto, Y., Ter Schure, E., Van Boven, A., Vankerckhoven, V., Zgoda, A., Tuijtelaars, S., Hansen, E.B., 2012. Food fermentations: microorganisms with technological beneficial use. Int J Food Microbiol 154(3) pp87-97
2. Campbell-Platt, G., 2003. Fermented foods – a world perspective. Food Res Int 27(3) pp253-257
3. Caplice, E., Fitzgerald, G.F., 1999. Food fermentations: role of microorganisms in food production and preservation. Int J Food Microbiol. 1999 50(1-2) pp131-49
4. FAO and WHO, 2001. Report on Joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria
5. FAO and WHO, 2002. Guidelines for the evaluation of probiotics in Food. Joint FAO/WHO Workgroup on Drafting Guidelines for the Evaluation of Probiotics in Food. London, Ontario, Canada, April 30 and May 1, 2002
6. Leuschner, R.-G.K., Robinson, T., Hugas, M., Cocconcelli, P.S., Richard-Forget, F., Klein, G., Licht, T.R., Nguyen-The, C., Querol, A., Richardson, M., Suarez, J.E., Vlak, J.M., von Wright, A., 2010. Qualified presumption of safety (QPS): a generic risk assessment approach for biological agents notified to the European Food Safety Authority (EFSA). Trends Food Sci. Technol. 21 pp 425-435
7. Magnússon, S.H., Gunnlaugsdóttir, H., Van Loveren, H., Holm, F., Kalogeras, N., Leino, O., Luteijn, J.M., Odekerken, G., Pohjola, M.V., Tijhuis, M.J., Tuomisto, J.T:, Ueland, O., White, B.C., Verhagen, H., 2012. State of the art in benefit-risk analysis: food microbiology. Food Chem Toxicol 50(1) pp33-9
8. Mogensen, G., Salminen, S., O’Brien, J., Ouwehand, A., Holzapfel, W., Shortt, C., Fonden, R., Miller, G.D., Donohue, D., Playne, M., Crittenden, R., Salvadori, B., Zink, R., 2002a. Food microorganisms – health benefits, safety evaluation and strains with documented history of use in foods. Bulletin of IDF 377 pp 4-9
9. Mogensen, G., Salminen, S., O’Brien, J., Ouwehand, A., Holzapfel, W., Shortt, C., Fonden, R., Miller, G.D., Donohue, D., Playne, M., Crittenden, R., Salvadori, B., Zink, R., 2002b. Inventory of microorganisms with a documented history of use in food. Bulletin of IDF 377 pp 10-19
10. Ross RP, Morgan S, Hill C., 2002. Preservation and fermentation: past, present and future. Int J Food Microbiol. 2002 79(1-2) pp3-16
11. Spano, G., Russo. P, Lonvaud-Funel, A., Lucas, P., Alexandre, H., Grandvalet, C., Coton, E., Coton, M., Barnavon, L., Bach, B., Rattray, F., Bunte, A., Magni, C., Ladero, V., Alvarez, M., Fernández, M., Lopez, P., de Palencia, P.F., Corbi, A., Trip, H., Lolkema, J.S., 2010. Biogenic amines in fermented foods. Eur J Clin Nutr. 64 Suppl 3 pp95-100
12. Stackebrandt, E., 2007. Forces shaping bacterial systematics. Microbe 2, pp283–288
13. Steinkraus, K.H., 2002. Fermentations in World Food Processing. Comp Rev Food Sci Food Safety [serial online]. 1 pp23-32
14. Vogel, R.F., Hammes, W.P., Habermeyer, M., Engel, K.H., Knorr, D., Eisenbrand, G.; Senate Commission on Food Safety (SKLM) of the German Research Foundation, 2011. Microbial food cultures–opinion of the Senate Commission on Food Safety (SKLM) of the German Research Foundation (DFG). Mol Nutr Food Res. 55(4) pp654-62
About the authors
François Bourdichon was deputy chair of the IDF Task Force ‘Update of Inventory of Microorganisms’ and is action team leader of the continuous update within the IDF Standing Committee on Microbiological Hygiene (SCMH) of IDF.
Joerg Seifert is the Technical Director of IDF and was in charge of coordination of outcomes of the task force with work program of IDF.
Egon Bech Hansen was Chair of the Task Force and is currently Head of the Department Administration DTU Systems Biology at the Technical University of Denmark.
Issue
Related topics
Related organisations
International Dairy Federation, Nestlé, Technical University of Denmark