Screening platform for optimal spray drying of enzymes and probiotics
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 19 June 2013 | Maarten Schutyser and Jimmy Perdana, Food Process Engineering Group, Wageningen UR, Martijn Fox, NIZO Food Research | 2 comments
Many food ingredients, such as enzymes and probiotics, are spray dried to provide a longer shelf life. A major hurdle when applying spray drying is the extensive optimisa tion required for formulation and drying conditions to obtain powders of acceptable quality. Therefore, a high-throughput screening platform based on single droplet drying mimicking spray drying was successfully developed. It allows, in combination with a novel viability enumeration technique, screening amongst others survival percentages of probiotic bacteria as a function of drying conditions and formulation…
Many food ingredients, such as enzymes and probiotics, are spray dried to provide a longer shelf life. A major hurdle when applying spray drying is the extensive optimisa tion required for formulation and drying conditions to obtain powders of acceptable quality. Therefore, a high-throughput screening platform based on single droplet drying mimicking spray drying was successfully developed. It allows, in combination with a novel viability enumeration technique, screening amongst others survival percentages of probiotic bacteria as a function of drying conditions and formulation...
Many food ingredients, such as enzymes and probiotics, are spray dried to provide a longer shelf life. A major hurdle when applying spray drying is the extensive optimisation required for formulation and drying conditions to obtain powders of acceptable quality. Therefore, a high-throughput screening platform based on single droplet drying mimicking spray drying was successfully developed. It allows, in combination with a novel viability enumeration technique, screening amongst others survival percentages of probiotic bacteria as a function of drying conditions and formulation.
Introduction
Spray drying is an effective and mild technique to stabilise and provide a longer shelf life to active ingredients in the form of powders. Very short drying times and relatively low temperatures have minimal effect on residual activity of heat sensitive ingredients. However, for heat sensitive products such as enzymes and probiotic bacteria, lyophilisation (freeze drying) is still preferred compared to spray drying. Because spray drying is much more cost effective as it can process larger volumes and operate at higher energy efficiencies, many attempts have been made to optimise spray drying and product formulations towards minimal activity losses. Although many successes have been reported, optimisation, for example on the basis of pilot-scale experiments, remains costly and problematic in practice1.
The optimal spray drying conditions vary strongly between different ingredients. For example, during spray drying, the survival of probiotics is found to increase with decreasing outlet temperatures and smaller residence times2. However, too low outlet temperatures may reduce drying efficiency and result in too high residual moisture content, leading to subsequent loss of viability during storage. Residence time is a critical parameter, which can vary considerably within different industrial spray dryers and possible following steps, such as fluidised bed drying at low temperatures. Other drying parameters of influence are concerned with the exact spray dryer configuration, such as nozzle type, positioning of air flow and injection of feed, and chamber design3. Besides optimal spray drying, appropriate formulation and sometimes specific pre-treatment procedures are also required to obtain products with extended shelf life4. Usually active ingredients are suspended in mixtures of carbohydrates to provide glassy, amorphous powders. It is advisable to use formulations with high glass transition temperatures as these are related to enhanced enzyme and microbial stability during shelf life. Finally, specific pre-treatments may be applied to enhance for example probiotic survival during spray drying and storage. Exposing bacteria to sub-lethal stress conditions (e.g. heat shock) prior to spray drying is a strategy to enhance survival percentages.
An attractive approach for screening of optimal spray drying conditions is via single droplet drying studies4. During single droplet drying, conditions such as drying air temperature, drying time, air flow rate and particle size can be controlled. Therefore, it is practical for model development and thus can be used to systematically investigate drying kinetics. Different single droplet drying modes have been employed to study drying kinetics such as acoustic levitation and droplets drying at the tip of a capillary. The single droplet drying method proposed by Wageningen University and NIZO Food Research involves drying of sessile droplets on a hydrophobic surface and has the main advantage that it allows a high-throughput screening approach.
Development and validation of the single droplet drying approach
A single droplet drying platform was successfully developed allowing the controlled drying of single sessile droplets5,6 (Figure 1). It starts with controlled pneumatic micro-dispensing of droplets with a diameter of 150 microns (minimum) on a hydrophobic surface. Subsequently, the sessile droplets are dried at a specific air temperature and relative humidity. Dried particles are harvested for further analyses. The drying behaviour of sessile water droplets was characterised in terms of heat and mass transfer and compared to drying of water droplets during industrial spray drying (Figure 2). Subsequently, the drying behaviour of maltodextrin suspensions was investigated and modelled using an effective diffusion model. The latter model predicts the developing temperature and moisture gradients inside the drying droplet. It was also proven that the contribution of heat conduction (via the contact between droplet and membrane) compared to the convective heat transfer via the air to the total evaporation could be neglected. As input for modelling of the drying behaviour, a dedicated approach was developed for measuring of moisture diffusivities in solid materials. This approach was based on gravimetric analysis of thin film drying in a dynamic vapour sorption (DVS) analyser. Results indicated a decrease of moisture diffusivity with decreasing moisture contents and temperatures. However, it also appeared that moisture diffusivities were similar for various carbohydrate matrices. Molecular interactions of e.g. hydroxyl groups seem to have a predominant influence on moisture diffusivity.
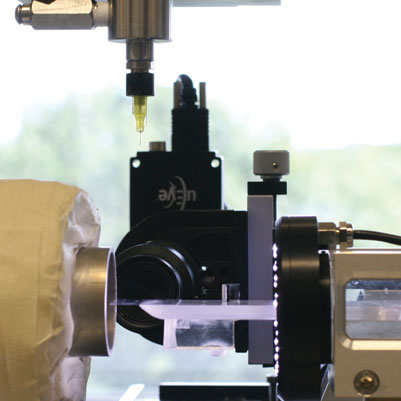

Figure 1: Single droplet drying platform with pneumatic dispenser, drying air channel, deposition platform and camera
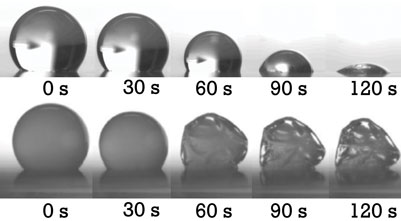

Figure 2: Time series of droplets drying comprising water only (top) and 20 w/w% maltodextrin DE6 (bottom)5,6
The enzyme b-galactosidase enzyme was selected as a model enzyme to verify our approach in assessing the influence of drying on inactivation of a heat sensitive ingredient during spray drying7. For this enzyme, we first characterised the effect of temperature and moisture content on activity loss (Figure 3). A mathematical model was developed to describe the inactivation kinetics of this enzyme as function of these two parameters. The latter inactivation kinetics was combined with the effective diffusion model to develop a predictive tool for the influence of drying on residual activity. This model was then used to reversely estimate the parameters of the inactivation kinetics from the single droplet drying experiments. Finally, the full inactivation kinetics was successfully verified by comparing model predictions and experimental data from laboratory-scale spray drying.
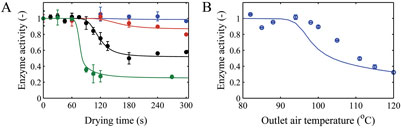

Figure 3: A) Measured and modelled enzyme inactivation as function of drying air temperatures of 80 (blue), 85 (red), 95 (black) and 110°C (green) during single droplet drying. B) Measured and predicted enzyme inactivation during laboratory-scale drying. The error bars indicate the standard deviation5,7
Optimal spray drying of probiotics
Probiotics are described as living microorganisms which, when administered in adequate amounts, confer a health benefit on the host. Health benefits are usually related to the influence of probiotic bacteria on the microbial balance in the hosts’ intestine or via modulation of the gut-associated immune system. Probiotics are often preserved by drying. Unfortunately, probiotics are mostly heat sensitive and therefore spray drying of these microorganisms is problematic because of low survival rates.
In our research, we chose Lactobacillus plantarum WCFS1 as the model organism for drying experiments. In collaboration with the Microbiology Group of Wageningen University (Dr. Ludmila Bereschenko and Professor Michiel Kleerebezem), a novel viability enumeration technique was developed and used to evaluate survival during drying8 (Figure 4). The method employs a micro-porous aluminium oxide chip (Anopore). (Semi-) dried particles are rehydrated on this micro-porous chip in a medium containing fluorescence probes for live/death enumeration. Subsequently, fluorescence-microscopy and image analysis were used to determine the live/dead ratio of bacteria. The method was found to provide survival percentages in agreement with conventional plating. Additionally, it has the advantage that it is compatible with a high-throughput approach. The novel viability enumeration method was successfully used in combination with single droplet and laboratory-scale spray drying experiments to study the effect of drying conditions on survival of L. plantarum WCFS1 (Figure 5). The influence of carrier formulations on residual viability was also investigated systematically and for example, large influences of glass transition temperature and the molecular weight of the solid carrier on survival were found. The latter is illustrated in Figure 5.
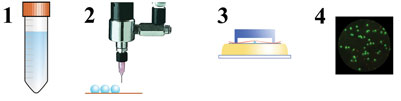

Figure 4: Schematic overview of the combined single droplet drying of probiotic bacteria and viability enumeration procedure. 1) microbial culture suspension, 2) single droplet drying, 3) reconstitution and staining, 4) fluorescence microscopy analysis8
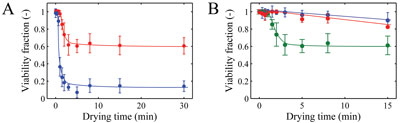

Figure 5: A) Viability fractions after single droplet drying at air temperatures of 25 (red) and 70°C (blue). B) Viability fractions after drying with different formulations of glucose (blue), trehalose (red), and maltodextrin DE6 (green) dried at 25°C. Initial dry matter was 20% w/w and initial droplet size was 600 μm. The error bars indicate the standard deviation and the solid lines are drawn to guide the eye8
The approach was subsequently used to study inactivation mechanisms of L. plantarum WCFS1 during drying. It appeared that two inactivation mechanisms could be distinguished. The first inactivation mechanism is dehydration inactivation, which occurs at all temperatures, due to destabilisation of the cell membrane during removal of water. The second inactivation mechanism is thermal inactivation (at temperatures above 45°C) and is related to loss of functionality of critical components, such as ribosomes or proteins in the cells. Surprisingly, it was found that during laboratory spray drying dehydration inactivation of L. plantarum WCFS1 was absent. This may be explained by the high drying rates in laboratory-scale spray drying (due to the smaller droplet size compared to industrial scale spray driers). Apparently, the fast drying leads to instant vitrification preventing destabilisation of the membrane. For slow drying processes (e.g. during freeze drying), relatively low survival rates were observed. The latter could again be explained by the dehydration inactivation during the slow drying (thus long drying times).
Conclusions
A screening platform was successfully developed for assessing the influence of drying and formulation conditions on heat sensitive ingredients. It was verified with the model enzyme b-galactosidase and further applied to study the influence of drying and formulation on the survival of L. plantarum WCFS1. In addition to the experimental platform, modelling approaches were also developed to extract inactivation kinetics from single droplet drying experiments. The obtained inactivation kinetics was successfully applied to predict inactivation and survival during laboratory-scale spray drying experiments. Future directions for research are to further develop the current platform for automated screening and apply it to assessing different powder functionalities as a function of drying and formulation parameters (e.g. morphology, surface properties related to wetting behaviour and encapsulation). If you are interested in our research or would like to participate in a consortium, don’t hesitate to contact us.
References
- P. Thybo, L. Hovgaard, J. Lindeløv, A. Brask, S. Andersen, Scaling Up the Spray Drying Process from Pilot to Production Scale Using an Atomized Droplet Size Criterion, Pharmaceutical Research, 25 (2008) 1610-1620
- J. Silva, R. Freixo, P. Gibbs, P. Teixeira, Spray-drying for the production of dried cultures, Int. J. Dairy Technol., 64 (2011) 321-335
- X. Zhou, J. Dong, J. Gao, Z. Yu, Activity-loss characteristics of spores of Bacillus thuringiensis during spray drying, Food and Bioproducts Processing, 86 (2008) 37-42
- M.A.I. Schutyser, J. Perdana, R.M. Boom, Single droplet drying for optimal spray drying of enzymes and probiotics, Trends in Food Science & Technology, 27 (2012) 73-82
- J. Perdana, M. Fox, M.I. Schutyser, R. Boom, Mimicking Spray Drying by Drying of Single Droplets Deposited on a Flat Surface, Food and Bioprocess Technology, 6 (2013) 964-977
- J. Perdana, M.B. Fox, M.A.I. Schutyser, R.M. Boom, Single-droplet experimentation on spray drying: Evaporation of a sessile droplet, Chemical Engineering and Technology, 34 (2011) 1151-1158
- J. Perdana, M.B. Fox, M.A.I. Schutyser, R.M. Boom, Enzyme inactivation kinetics: Coupled effects of temperature and moisture content, Food Chemistry, 133 (2012) 116-123
- J. Perdana, L. Bereschenko, M. Roghair, M.B. Fox, R.M. Boom, M. Kleerebezem, M.A.I. Schutyser, A novel method for viability enumeration for single-droplet drying of Lactobacillus plantarum WCFS1, Applied and Environmental Microbiology, 78 (2012) 6
Biographies
Maarten Schutyser obtained his PhD degree (2003) cum laude at Wageningen University. He worked for six years in industrial R&D. Since 2008, he has been an assistant professor at the Food Process Engineering Group. His research group focuses on drying and other separation technologies for efficient production of food ingredients. Maarten Schutyser chairs the Dutch Working Group on Drying.
Jimmy Perdana obtained his MSc degree Food Technology cum laude in 2009 at Wageningen University via the prestigious Huygens Scholarship. In the same year, he joined the Food Process Engineering Group to start a PhD project on the development of a high throughput screening platform to study spray drying of probiotics.
Martijn Fox obtained his PhD degree (2006) at Wageningen University. He joined NIZO food research as a project manager and his expertise is concerned with heating, drying and evaporation processes. His work varies from applied research to optimisation and troubleshooting of processes. Martijn Fox hosts the annual NIZO course on evaporation and spray drying.




Great article.
I have interest on this class of information