A perspective on actual technologies and future developments
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 18 August 2008 | Giuseppe Mensitieri, University of Naples Federico II and Giovanna Giuliana Buonocore, Institute for Composite and Biomedical Materials – National Research Council | No comments yet
In this contribution, the topic of Modified Atmosphere Packaging (MAP) is reviewed by describing the actual status of this technology and its possible developments, which are mainly related to the combination of MAP with other preservation technologies. Among them, particular attention is devoted to active packaging with antimicrobial properties.
In this contribution, the topic of Modified Atmosphere Packaging (MAP) is reviewed by describing the actual status of this technology and its possible developments, which are mainly related to the combination of MAP with other preservation technologies. Among them, particular attention is devoted to active packaging with antimicrobial properties.
In this contribution, the topic of Modified Atmosphere Packaging (MAP) is reviewed by describing the actual status of this technology and its possible developments, which are mainly related to the combination of MAP with other preservation technologies. Among them, particular attention is devoted to active packaging with antimicrobial properties.
Modification of head space atmosphere in packaging (MAP), in terms of total pressure and/or partial pressure of gaseous components, is an effective technology to extend the shelf life of most packaged food products. By properly tailoring the modified atmopshere, high improvement in the preservation of foods can be obtained due to the inhibition of enzymatic or microbial action, reduction of chemical oxidation and of physical processes (Lee et al, 2008).
In this communication, we will focus on the effects of compositional changes of starting head space atmosphere also in relationship with other novel technologies such as active packaging, while we will not cover the aspects related to vacuum, hypobaric and hyperbaric packaging.
Modified atmosphere can be either actively imposed by introducing in the packaging headspace a gaseous mixture of a selected composition or achieved passively by the natural evolution of starting internal atmosphere due to interaction with food and permeation through the package wall. The evolution of the internal atmosphere can also be modulated as a consequence of the action of active substances, such as gas scavengers or gas release devices, and its beneficial action can in principle be enhanced by coupling with antimicrobial packaging.
MAP gases
Most common gaseous mixtures for MAP are based on nitrogen, oxygen and carbon monoxide but, depending on local legislation, the use of other gases is allowed, such as argon, hydrogen, helium, nitrous oxide and carbon monoxide. The effectiveness of the gas depends on its interaction with food components and with packaging material and should be evaluated by also considering the synergic action deriving from a combination of different gases. In the following article, the main gases adopted in MAP are briefly reviewed.
Oxygen
Although the presence of oxygen in the internal atmosphere is not considered beneficial for food preservation since most of the food spoilage bacteria, yeasts and moulds are aerobic, oxygen is often necessary to avoid dangerous anaerobic conditions, to guarantee aerobic respiration in fresh fruits and vegetables or to guarantee a bright colour in red meats.
Carbon dioxide
The action of CO2 is favoured by its high solubility in fatty and high water content foods. It slows down, in particular, the microbial deterioration promoted by Gram-negative, aerobic spoilage bacteria and moulds by different possible mechanisms of action (Stiles, 1991; Farber, 1991) and at levels above one per cent can render plant tissues insensitive to the ripening hormone ethylene (Lee et al, 2008). Since the solubility of carbon dioxide increases as temperature decreases, a low temperature of the packaged food is essential for the efficiency of carbon dioxide in retarding microbial spoilage. Restrictions to a high concentration of CO2 is determined by undesired effects, for example, package collapse due to reduction of head space volume, exudation of flesh foods and surface discoloration of food.
Nitrogen
This gas, per se, displays negligible antimicrobial activity, but it is used to dilute O2 and CO2 to limit deleterious effects determined by these gases.
Noble gases
The noble gases are a family of elements characterised by their lack of reactivity and include helium, argon, xenon and neon. These gases are being used in a number of food applications, e.g. potato-based snack products. On scientific grounds it is difficult to see how the use of noble gases would offer any preservation advantages compared with N2.
Carbon monoxide
Potential applications of CO in MAP is aiming to guarantee a bright red colour to meat, to inhibit several browning reactions in fruits and vegetables acting also as a fungistat and as an antimicrobial against common contaminant even at a concentration of one per cent (Lee et al, 2008). However, CO can cause possible health hazards to operators of packaging machines and is not an approved food additive in USA and Europe, while in Canada and Japan its use is prohibited for fresh meats and fishes (Lee et al, 2008). However, attractive results obtained with CO are determining controversial decisions, although the Scientific Committee on Food of the European Commission opposed the use of CO in MAP (Otwell et al, 2003).
Sulphur oxide
It is anti-microbial in its unbound, non-ionised molecular form and is used (generally at pH<4) to prevent the growth of bacteria and mould on soft fruits and microbial growth in juices and wines. Its action is selectively dependent on concentration and is most effective against Gram-negative bacteria (Lee et al, 2008).
Microbiological aspects
Growth and reproduction of microorganisms require specific conditions in terms of intrinsic factors of food (e.g. water activity and pH) and extrinsic factors such as temperature and the gaseous composition of the environment. MAP simply acts by retarding spoilage without enhancing product quality and, to be effective, should always be coupled with best quality and safety management.
Spoilage microorganisms
Spoilage microorganisms compromise food quality and hygienic safety promoting changes in texture, colour, flavour and odour. Concentrations of CO2 above five per cent v/v inhibit the growth of most spoilage bacteria (more effective for Gram-negative bacteria).
Pathogenic microorganisms
The effect of MAP on food pathogens is rather incomplete, although high levels of CO2 have been found to have an inhibitory effect on Staphylococcus aureus, Salmonella spp, E. coli and Y. entrolitica (Lee et al, 2008). Since there are several pathogenic bacteria capable of growth at approximately 5°C or below, the ability of MAP to inhibit their growth in foods under refrigerated storage is extremely important. In particular, C. botulinum type E is of great concern since it is both anaerobe and low-temperature tolerant and can produce toxin at low temperatures in oxygen-free MAP atmospheres before spoilage symptoms can be detectable by the consumer. It is generally accepted that MAP containing at least two per cent oxygen should provide an adequate safeguard for products susceptible to its contamination.
An initial high quality product without contamination by any pathogenic microorganisms is a prerequisite for successful MAP and, in general, MAP should always be coupled with good hygienic practice and controlled temperature. In fact, application of MAP is preferentially devoted to the preservation of low water content or sterilised foods coupled to low temperature storage.
MAP atmospheres and food products
MAP can improve the preservation of different classes of foods including fresh produce, meats, fishes and bakery products. In the following MAP, gas mixtures compositions are briefly reviewed with reference to non-respiring and respiring products.
Non-respiring products
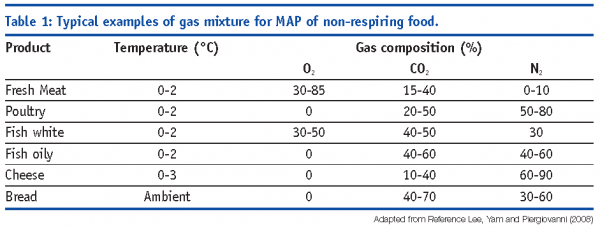
In the case of non-respiring products, the starting headspace atmosphere composition should be tailored on the basis of specific deterioration processes and interaction with head space to guarantee the optimal preservation (see Table 1 for some examples [Lee et al, 2008]).
Bakery products that are susceptible to spoilage by bacteria, yeasts and moulds can have their shelf lives extended by MAP with high CO2 content, although costs limit this application. The most common forms of deterioration are microbiological spoilage, staling and moisture loss or gain and use of CO2 has an inhibitory effect on the growth of most microorganisms and is suitable for food preservation on stability, toxicity, organoleptic and economic grounds. To avoid packaging collapse problems encountered with high CO2 contents, mixtures of CO2 and N2 are generally adopted. Mould-free shelf life has been proven to increase with the percentage of carbon dioxide in the head space atmosphere, in an amount dependent on water activity of the food produce (Seiler, 1999). Although carbon dioxide is also effective against aerobic bacteria, anaerobic bacteria could develop and cause rope spoilage which could be, however, limited by adding permitted preservatives. Growth of yeasts by post-baking contamination is difficult to inhibit by using MAP, even with high CO2 concentration. This evidence stresses again the need for high attention to appropriate hygienic procedures. Finally, modifying headspace atmosphere with ethanol vapour is an excellent way of preserving bakery products as it limits moulds, yeasts, microbial contaminants and prevents staling.
MAP can also be used with good results for cheese. For hard cheeses, the optimum mixture has been claimed to be 75 per cent CO2 – 25 per cent N2 (Berne, 1994), for unripened cheeses, where the primary cause of spoilage is the growth of psychrotrophs and Gram-negative bacteria, MAP has become an interesting alternative to the use of preservatives and CO2/N2/O2 mixtures have been successfully adopted with cottage cheese, achieving inhibition of yeast and mould growth.
N2 flushing has been reported to be effective in the preserving quality of yoghurt and milk, coffee beans and ground coffee (to replace vacuum packaging in valve packs which allows the CO2 to leave the head space and impeding the ingress of O2 from the outside), snacks (to increase the oxidation stability) and fruit juices.
MAP extends the shelf-life of most fishery products by inhibiting bacterial growth and oxidative reactions. The achievable extension of shelf-life depends on species, fat content, initial microbial population, gas mixture, the ratio of gas volume to product volume and, most importantly, storage temperature: without proper control of storage temperature, the benefits of MAP may be lost. The CO2 is usually mixed with N2 which acts as inert filler and with O2 which is used in quite high concentration in order to reduce the bacterial spoilage flora and the potential risk for botulism in the sealed pack. However, optimal gas mixture composition may vary considerably, mainly with reference to carbon dioxide and oxygen content.
For red meat, gaseous mixtures of interest for MAP include O2, CO2 and N2. Oxygen is used to maintain the red colour retarding the brown discoloration. CO2 is used to delay aerobic deterioration while N2 prevents the collapse of the package as CO2 is dissolved into the meat.
Respiring products
Typical headspace gas concentrations for the maintenance of best quality for fruits and vegetables may range from two to 10 per cent oxygen and 10 to 20 per cent carbon dioxide for cut produce. A high carbon dioxide concentration should be avoided in order to avoid the shift to anaerobic bacteria population and resident microflora would develop considerably, leading to rapid spoilage. Proper film permeability, healthy resident microflora and optimal refrigeration storage temperature act synergistically to guarantee the safety of the fresh-cut produce.
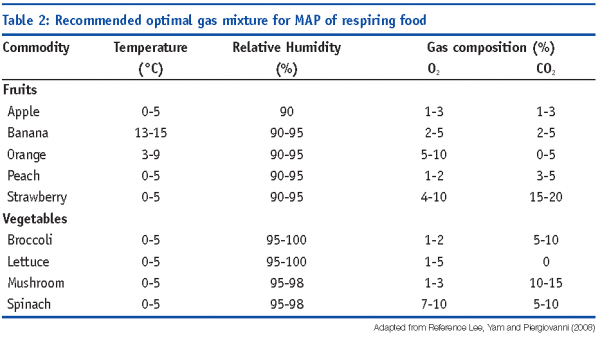
MAP with reduced O2 and elevated CO2 concentrations in fresh products reduces respiration rate, ethylene production and texture softening. O2/CO2 mixtures (80-90 per cent / 20-10 per cent) have been proposed to suppress microbial growth in minimally-processed vegetables. Some examples of optimal MAP conditions are reported in Table 2 (Lee et al, 2008).
For mould-ripened and soft cheeses, the packaging of these products under MAP is more complicated than MAP of hard cheeses owing to the presence of live moulds, since the mould growth should be allowed to continue at a controlled rate, hence oxygen cannot be totally excluded from the headspace. Typically, gas mixtures containing 20-40 per cent of CO2 are adopted.
The evolution of the MAP head space composition
While a specific optimal atmosphere might be achieved initially, composition of the headspace atmosphere evolves during the life of packaged products as a consequence of physical (for example, gas sorption) and chemical phenomena (respiration) and of mass exchange across the thickness of packaging material under the driving force of the different gas partial pressure inside and outside the package. Hence the success of a MAP is based on a proper design and modelling of processes occurring inside the package (Talasila et al, 1997; Rotabakk, 2008)
Non-respiring products
Non-respiring products have a limited effect on headspace atmosphere composition. In fact CO2 sorption and consumption and some O2 production can still occur, but are rather limited and promote only negligible effects on the headspace gas mixture composition, which changes mainly as a consequence of permeation and sorption properties of packaging material.
As a consequence, application of MAP for this class of foods would require the use of packaging materials with the highest barrier possible and imposing as starting head space atmosphere, the one that guarantees the best preservation. Another important factor is the ratio between the amount of gas and the amount of product.
Respiring products
In the case of respiring products, headspace atmosphere tends to evolve. In this case permeability of packaging materials should be properly chosen to maintain the O2 and CO2 concentration within tolerance limits. In fact, O2 is consumed and CO2 is produced in a ratio which is called respiratory quotient (RQ) which depends upon the type of substrate. The respiration also produces water and, in some cases, enzymatic reactions produce ethylene which in turn accelerates respiration reactions. Moreover, CO2, water and ethylene permeate from inside to outside the packaging and O2 from outside to inside.
Designing MAP for respiring products (Robertson, 1992) requires adequate modelling of respiration kinetics and permeation rate through the package. As an example, in the case RQ=1, the following relationship relates the O2 concentration ([O2]) and the CO2 concentration ([CO2])which establish in steady state conditions inside the head space to the ratio of O2 and CO2 permeability (PCO2/PO2) of the packaging materials:
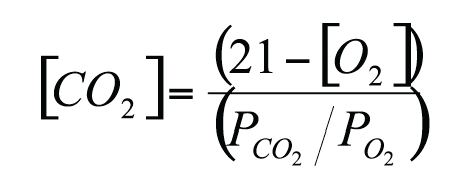
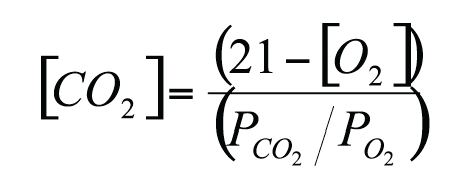
For a fixed permeability ratio, the gas concentrations depend upon the relative amount of food per package volume and on temperature (carbon dioxide concentration increases and oxygen concentration decreases with increasing food weight and increasing temperature). The time needed to reach steady state conditions depends upon the headspace volume, increasing as the volume increases.
Packaging materials for MAP
Due to their excellent barriers, glass and metal containers are potentially suitable for MAP application with non-respiring products but, generally, the quality of foods processed and packed in these containers are not enhanced by gas introduction.
Concerning plastic materials, both semi-rigid containers and flexible films are generally suitable for MAP applications. Main properties to be considered for these types of materials are the type of package, the barrier properties, the machinability, the mechanical strength, sealability and printability. Barrier properties of plastic materials range from high barrier materials to high permeability materials offering a wide choice in the following polymer categories: polyolefins, vinyl polymers, polyamides and polyesters. Different properties can be obtained by combining films of different nature in multilayer structures realised by coextrusion, lamination or coating processes. An analysis of packaging materials for MAP can be found in the book edited by Blakistone (1999).
Non-respiring products
MAP applications for non-respiring food products demand a high barrier to oxygen, carbon dioxide and moisture. Plastic packaging used for this aim are made of multilayer (laminated or coextruded) high barrier structures generally containing ethylene-vinyl-alcohol (EVOH) , polyvinylidenechloride (PVdC), polyethyleneterphthalate (PET), nylon and high density polyethylene (HDPE).
Respiring products
To maintain the optimal head space modified atmosphere, permeation to gases of the packaging materials and head space volume should be balanced in accordance with the reactions occurring inside the package which consume and produce different chemical species (Al-Ati et al, 2003). Packaging materials should be selected mainly on the basis of O2 and CO2 permeability and of their temperature dependence. In most polymeric films, PCO2/PO2 ranges between three and six. This is obviously a limitation which could be circumvented by inserting windows of different materials or by perforating (Lee et al, 2000; Paul et al, 2002) the package structure, attaining higher or lower effective permeability ratios. It is worth noting that the activation energy for permeation phenomena through packaging materials is generally lower than that typical of respiration: a temperature change with respect to temperature assumed in design of MAP can lead to a shorter shelf life than expected.
Actual trends and future developments in MAP technology
Future development and efficacy improvement of MAP will likely be obtained by exploiting the combination between this technology and other preservation techniques (Sivertsvik et al, 2002; McMillin, 2008). The proper choice of storage temperature, water activity, pH, redox potential and preservatives, bioconservation, possible application of ultrahigh pressure treatment, use of edible, antimicrobial and other active packaging systems could be beneficial in combination with MAP to attain longer shelf lives.
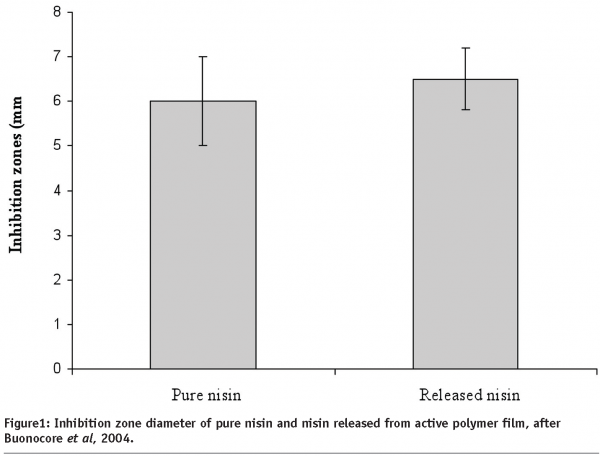
In particular, a promising research filed is the development of antimicrobial films in which the active compound is initially incorporated in the packaging films structure and then released into the food or obtained by creating, on the packaging film surface in contact with food, species (e.g. amines) with antimicrobial action. Several antimicrobial films have been developed in the past, but the release rate and migration profile of the antimicrobial agents were not specifically controlled. To this aim, Buonocore et al (2003) successfully proposed to control the release of three active compounds (lysozyme, nisin and sodium benzoate) by using the degree of crosslinking of an highly swelling polymer matrix or by using a multi-layer structure, developing packaging systems designed to avoid the occurrence of loss of antimicrobial activity (Buonocore et al, 2004). As an example, the effectiveness of nisin was tested on Alicyclobacillus acidoterrestris spores (a potential spoilage concern in hot-fill fruit and vegetable juices) by determining the evolution cell loads in presence of the nisin-containing film. In Figure 1, the inhibition zone diameter of pure and released nisin is reported. No significant differences are observed, thus suggesting that nisin released from the active film does not lose its efficiency as antimicrobial agent.
The synergistic effect of antimicrobial and MAP has been recently investigated by Lopez-Mendoza et al (2007) showing that the use of MAP by itself only determined a bacteriostatic effect, but the combination of MAP with the antimicrobial compounds achieved a relevant bactericidal effect for raw ground pork.
MAP can be used in combination with other preservation treatments to extend the shelf-life of packaged products: heat processing and irradiation have beneficial effects on food produce preservation. CO2 scavengers and ethylene absorbers can be used to prevent a potentially dangerous increase in concentration of these substances inside the MAP package. In addition, humidity absorbers are sometimes used in MAP to avoid moisture condensation.
The development of ‘smart’ packaging films characterised by a temperature dependence of gas permeability which mimics that of the degradation processes of packaged food is also of interest. Finally, smart packaging, such as Time Temperature Indicators (TTI) is a technology that appears to have a good potential in combination with MAP for chill-stored products.



References
Al-Ati, T., Hotchkiss J.H. (2003) The Role of Packaging Film Permselectivity in Modified Atmosphere Packaging, J. Agric. Food Chem., 51, 4133-4138.
Berne S., (1994) Mapping the future with CAP-ability’, Prepared Foods, 163, 101-10.
Blakistone B.A. (1999) ed. ‘Principles and Applications of Modified Atmosphere Packaging of Foods’, 2nd ed., Aspen Publishers Inc., Gaithersburg, Maryland.
Buonocore, G.G., Del Nobile, M.A., Panizza, A., Corbo, M.R., Nicolais, L. (2003) A general approach to describe the antimicrobial Agent Release from highly swellable films intended for food packaging applications, Journal of Controlled Release, 90, 97-107.
Buonocore, G.G., Sinigaglia, M., Corbo, M.R., Bevilacqua, A., La Notte, E., Del Nobile, M.A. (2004) Antimicrobial Release Systems from Highly Swellable Polymers Journal of Food Protection, Vol.67, No.6, 1190-1194.
Farber J.M. (1991). Microbiological aspects of modified-atmosphere packaging technology: a review, J. Food Prot., 54, 58-70.
Lee, D.S., Kang, J.S., Renault, P. (2000) Dynamics of internal atmosphere and humidity in perforated packages of peeled garlic cloves, Int. J. Food Sci. Tech., 35, 455-464.
Lee, D.S., Yam, K.L Piergiovanni, L. (2008). Food Packaging Science and Technology, CRC Press, Boca Raton Fl.
Lopez-Mendoza, M.C., Ruiz, P., Mata, C.M. (2007) Combined effects of nisin, lactic acid and modified atmosphere packaging on the survival of Listeria monocytogenes in raw ground pork. Int. Journal of Food Science and Technologies. 42, 562-566.
McMillin, K.W. (2008) Where is MAP Going? A review and future potential of modified atmosphere packaging for meat’, Meat Science, 80, 43-65.
Otwell, W.S., Balaban, M., Kristinsson, H. (2003). Use of carbon monoxide for color retention in fish, in Conference Proceedings First Joint Trans-Atlantic Fisheries Technology Conference – TAFT 2003, 33rd WEFTA and 48th AFTC meetings. 2003, Reykjavik, Iceland.
Paul, D.R., Clarke, R. (2002) Modeling of modified atmosphere packaging based on designs with a membrane and perforations’, J. Membrane Sci., 208, 269-283.
Rotabakk, B.T,. Wyller, J., Lekang, O.I., Sivertsvik, M. (2008) A mathematical method for determining equilibrium gas composition in modified atmosphere packaging and soluble gas stabilization systems for non-respiring foods, J. of Food Eng., 85, 479-790..
Seiler D.A.L., (1999) Bakery products’, in B.A. Blakistone ed. ‘Principles and Applications of Modified Atmosphere Packaging of Foods’, 2nd ed., Aspen Publishers Inc., Gaithersburg, Maryland.
Sivertsvik, M., Jeksrud, W. K., Rosnes, J. T. (2002) A review of modified atmosphere packaging of fish and fishery products – significance of microbial growth, activities and safety, Int. J. of Food Sci. and Tech., 37, 107-127.
Talasila, P.C., Cameron, A.C. (1997). ‘Prediction equations for gases in flexible modified atmosphere packages of respiring produce are different than those for rigid packages’, J. Food Sci., 62, 926-930.
Issue
Related topics
Packaging & Labelling, Quality analysis & quality control (QA/QC)







