Low-density lipoproteins from egg yolk: A natural carrier of highly emulsifying species
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 28 February 2013 | Marc Anton, INRA, UR1268 Biopolymères Interactions Assemblages | 1 comment
Hen egg yolk low-density lipoproteins (LDL), natural nanoemulsions of 30 nanometres in diameter, are the main contributors to egg yolk interfacial and emulsifying properties. These properties are clearly due to the LDL structure through interactions between amphiphilic apoproteins and phospholipids. This structure allows transport through the aqueous phase and until the interface of these amphiphilic species that would be insoluble in another form. The lipoproteins disrupt at the oil-water interface and then interfacial films made with LDL are constituted by a blend of apoproteins and phospholipids that assure both the decrease on interfacial tension and the resistance to rupture. This permits the formation and the stability of food emulsions made with yolk. Additionally, LDL could be used as natural or modified nanocapsules to protect, carry, deliver and finally increase the bioavailability of micronutrients.
Hen egg yolk low-density lipoproteins (LDL), natural nanoemulsions of 30 nanometres in diameter, are the main contributors to egg yolk interfacial and emulsifying properties. These properties are clearly due to the LDL structure through interactions between amphiphilic apoproteins and phospholipids. This structure allows transport through the aqueous phase and until the interface of these amphiphilic species that would be insoluble in another form. The lipoproteins disrupt at the oil-water interface and then interfacial films made with LDL are constituted by a blend of apoproteins and phospholipids that assure both the decrease on interfacial tension and the resistance to rupture. This permits the formation and the stability of food emulsions made with yolk. Additionally, LDL could be used as natural or modified nanocapsules to protect, carry, deliver and finally increase the bioavailability of micronutrients.
Hen egg yolk low-density lipoproteins (LDL), natural nanoemulsions of 30 nanometres in diameter, are the main contributors to egg yolk interfacial and emulsifying properties. These properties are clearly due to the LDL structure through interactions between amphiphilic apoproteins and phospholipids. This structure allows transport through the aqueous phase and until the interface of these amphiphilic species that would be insoluble in another form. The lipoproteins disrupt at the oil-water interface and then interfacial films made with LDL are constituted by a blend of apoproteins and phospholipids that assure both the decrease on interfacial tension and the resistance to rupture. This permits the formation and the stability of food emulsions made with yolk. Additionally, LDL could be used as natural or modified nanocapsules to protect, carry, deliver and finally increase the bioavailability of micronutrients.
LDL structure and composition
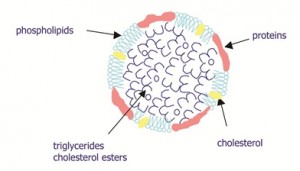
Yolk represents about 36 per cent of the weight of fresh whole egg. It is composed of about 50 per cent water, 32 per cent lipids, 16 per cent proteins, one per cent carbohydrates and one per cent ashes[1]. On the basis of its dry matter, yolk is constituted of 68 per cent low-density lipoproteins (LDL), 16 per cent highdensity lipoproteins (HDL), 10 per cent globular protein: livetin, four per cent phosphoprotein: phosvitin, and two per cent minor proteins (Figure 1, page 28). Considering our topic, yolk is composed of two kinds of supramolecular assemblies differing in their size and their composition: LDL and granules. LDL (two thirds of yolk dry matter) are spherical molecules (17 – 60 nanometres diameter) with a lipid core (triglycerides and cholesterol esters) surrounded by a phospho – lipid and protein film[2] (Figure 2, page 29). Phospholipids take an essential part in the stability of the LDL structure because association forces are essentially hydrophobics[3]. LDL are soluble in aqueous solution whatever the medium conditions due to their low density (0.982 g / ml). LDL contains 83 – 89 per cent lipids and 11 – 17 per cent proteins. Lipids are composed of about 69 per cent triglycerides, 26 per cent phospholipids and five per cent cholesterol. Consequently, the ratio of phospho – lids and proteins in LDL is approximately 1.5:2. Proteins of LDL are composed of six apoproteins[4-6]. The major apoprotein accounts for more than 70 per cent of the apoproteins. Its molecular weight is estimated to be 130,000 Dalton. The second apoprotein (15-20,000 Dalton) represents about 20 per cent of the apoproteins. Four other minor apo proteins with molecular weights between 60,000 and 95,000 Dalton have been identified. Apoproteins of LDL contain about 40 per cent of hydrophobic amino acids and present a random coil structure or a β- sheet conformation[7,8]. Consequently, they are highly hydrophobic and flexible molecules.
Lipids, the primary components of yolk (64 per cent), are distributed exclusively in the lipoproteins (HDL and LDL). They are composed of triglycerides (65 per cent), phospho lipids (29 per cent), cholesterol (five per cent), free fatty acids (less than one per cent), and others including carotenoids (less than 0.1 per cent). Phospholipids of yolk are very rich in phosphatidylcholine (PC), which makes 76 per cent of total phospholipids. PC is a zwitterionic phospholipid over a pH range from strongly acid to strongly alkaline. Phospha – tidylethanolamine represents only 22 per cent of phospholipids. Phosphatidylinositol (PI), phosphatidylserine (PS), sphingomyelin (SM), cardiolipins (CL), lysoPC, and lysoPE are present in very low amounts. PUFA represent 30 – 40 per cent of the fatty acids, whereas SFA account for 45 per cent and MUFA for 20 – 25 per cent. The saturated fatty acids are essentially located in sn-1 and the unsaturated fatty acids in the sn-2 position of phospholipids[9].


Figure 1 Constituents of plasma and granules from hen egg yolk
(LDL: low-density lipoprotein, HDL: high-density) lipoprotein
LDL adsorption and emulsifying properties
The emulsifying properties of egg yolk are principally attributed to LDL. They contribute to the formation and the stabilisation of food emulsions by their emulsifying activity and stability.
Emulsifying activity is related to the capacity of surface active molecules to cover the oil-inwater interface created by mechanical homog – enisation, and to reduce the interfacial tension. Emulsion stability indicates the capacity to avoid flocculation, creaming and/or coalescence of oil droplets. Creaming and flocculation are reversible phenomena which can be avoided by a simple agitation of the emulsion. Coalescence is the irreversible fusion of oil droplets due to the rupture of the interfacial film created by emulsifying agents. This phenomenon leads to complete destruction of the emulsion.
In food emulsions, there are two major types of emulsifying agents: low-molecular weight surfactants (mono- and di-glycerides, phospholipids, etc) and macromolecular surfactants (proteins). Generally, low-molecular weight surfactants provide a smaller interfacial tension than macromolecular surfactants, and consequently have a better emulsifying activity. Conversely, macromolecular surfactants usually form interfacial film more resistant to coalescence than low-molecular weight surfactants, and consequently, they are better emulsion stabilisers. In food emulsions, a mixture of the two types of emulsifying agents are used, and in egg yolk, these two types of emulsifying agents co-exist, forming supra – molecular assemblies (lipoproteins) due to their amphiphilic characteristics.
The structure of LDL seems essential to ensure their interfacial properties, as any denaturing treatment affects their emulsifying properties[10,11]. Mizutani and Nakamura[12] demonstrated that increasing treatments of LDL by proteases (Trypsin and papain) produced a decrease of their properties of formation and stabilisation of emulsions. It was then suggested that only a small amount of the phospholipids of LDL take part in the adsorption at the oil-water interface and that the protein part of LDL played the essential role6. More recently[13-15], we have confirmed the driving contribution of the protein aceous part of yolk, especially apoprotein of LDL, in the formation and stability of emulsions made with yolk.
It is commonly supposed that LDL particles break down when they come into contact with the interface. The lipid core coalesces with the oil phase and apoLDL and phospholipids spread at the interface[16-18]. The disruption of LDL particles is attributed to a weakening of protein-protein interactions.
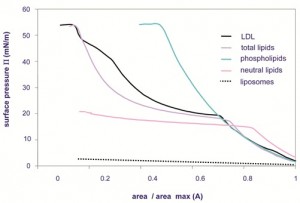

Figure 3 Π/A isotherms of LDL, total lipids, phosphlipids and liposomes. Lipids are extracted from purified LDL
suspension and consist of neutral lipids (spread amount=85 μg), phospholipids (spread amount=198 μg) and total
lipids, mixture of the two previous species (spread amount=287 μg)
Direct adsorption of LDL apoproteins is not easy because of the non solubility of these apoproteins in water or in aqueous buffer. The interactions between apoproteins and lipids in LDL are essential to transport surfactant apoproteins in a soluble form (LDL particles) at the neighbouring of the interface and then to release them at the interface. The interactions between apoproteins and phospholipids before adsorption seem to be the key factor to explain LDL emulsifying properties.
Using Langmuir film balance (air-water interface), we have detected three phase transitions in compressor isotherms and we have attributed these three transitions to neutral lipids, apoproteins and phospholipids, respectively (Figure 3). This showed that LDL have to break down when they come into contact with the interface to release neutral lipids, phospholipids and apoproteins from the lipoprotein core and allow their spread. So we can observe that LDL serves as a vector of surfactant constituents (apoproteins and phospholipids with hydrophobic interactions) that could not be soluble in water but that can adsorb once they are transported near an interface. Considering these previous results, the influence of each lipids class present in the LDL structure has been identified in the overall LDL surface behaviour. The lipid constituents behave in a relatively independent and indi – vidual way within the film after spreading of LDL suspension at the air-water interface.
Recent studies conducted in our lab – oratory[19,20] demonstrated, using extracted phospholipids or apoproteins, or recombined apoproteins / phospholipids, that the respective role of phospholipid and apoprotein in LDL interfacial properties is modulated by the pH value. Compressor isotherms of recombined apoproteins/ phospholipids, obtained in Langmuir film balance, showed that at pH 3, the influence of phospholipids seems predominant whereas at pH 7.5 the impact of apoproteins is more important. Furthermore, the organisation of phospholipids is essential. When phospholipids are used in aqueous phase, they decrease interfacial tension very slightly (γeq: 20 mN/m), whereas added to the oil phase or organised in small unilamellar vesicule (SUV), they are more efficient (γeq: 15 mN/m), whatever the pH value. Finally, when recombination of phospho – lipids/apoproteins was used, values near those obtained with native LDL were observed (2-5 mN/m). This confirms that both apoprotein and phospholipid parts are essential to understand interfacial properties of yolk LDL.
Using atomic force microscopy (AFM) after a Langmuir-Blodgett transfer of the layers from the air-water interface to a silica plate, we have shown that the second transition (previously attributed to apoproteins alone) should not be due to apoproteins alone, but to apoproteinslipids complexes14,15. So we have deducted that LDL serve as vectors of surfactant constituents (apoproteins and phospholipids) that could not be soluble in water, until the interface. At this step, the conservation of the LDL structure is essential. Once LDL are near the interface, the structure is then broken up to release surfactant constituents at the interface (Figure 4).
Furthermore, we have shown, comparing interfacial behaviour of LDL and liposomes (double phospholipid layer not containing proteins), that the apoproteins situated on the LDL surface start the LDL disruption mechanism by their initial anchorage. The compression isotherm confirmed that the liposomes made only with phospholipids extracted from LDL do not spread at the air-water interface, whereas, with proteins at the LDL surface, the anchorage provokes an unfolding of the protein, leading to the destabilisation of the external layer of the LDL. Then this phenomenon could be followed by a deformation of the particle due to the creation of a neutral lipid lens conducing to the spreading of the LDL constituents. In the case of liposomes, without external proteins the structure remains steady at the interface and then this structure is not able to adsorb efficiently and to decrease interfacial tension.
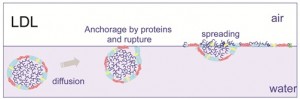

Figure 4 Hypothetical mechanism of LDL adsorption at an oil-water interface as compared with
liposome behaviour
Conclusion
LDL from yolk can be characterised as natural nano-emulsions carrying very efficient sur – factants species that could not be soluble in water. This explains the amazing emulsifying capacities of egg yolk and its fractions. This knowledge will serve to develop new ingredi – ents from yolk (plasma) with targeted properties like nutrient enrichment, for example.
References
- Powrie WD, Nakaï S. The chemistry of eggs and egg products. In Stadelman WJ, Cotterill, OJ, eds. Egg Science and Technology. Westport: The Avi Publishing Company, pp 97-139, 1986
- Cook WH, Martin WG. Egg lipoproteins. In: Tria E, Scanu AM, ed. Structural and Functional Aspects of Lipoproteins in Living Systems. New York: Academic Press, pp 579-615, 1969
- Banaszak LJ, Ross JM, Wrenn RF. Lipovitellin and the yolk lipoprotein complex. In: Jost PC, Griffith OH, eds. Lipid-protein interactions. New York: John Wiley & Sons, pp 233-258, 1982
- Nakamura R, Hayakawa R, Sato Y. Isolation and fractionation of the protein moiety of egg yolk low density lipoprotein. Poultry Sci 56:1148-1152, 1977
- Anton M, Gandemer G. Composition, solubility and emulsifying properties of granules and plasma from egg yolk. J Food Sci, 62:484-487, 1997
- Le Denmat M, Anton M, Beaumal V. Characterisation of emulsion properties and of interface composition in oil-in-water emulsions prepared with hen egg yolk, plasma and granules. Food Hydrocolloids 14 : 539-549, 2000
- Anton M, Martinet V, Dalgalarrondo M, Beaumal V, David-Briand E, Rabesona H. Structural and chemical characterisation of low-density lipoproteins purified from hen egg yolk. Food Chem, 83:175-183, 2003
- Jolivet P, Boulard C, Chardot T, Anton M. New insights into the structure of apolipoprotein B from lowdensity lipoproteins and identification of a novel YGP-like protein in hen egg yolk. J Agric Food Chem, 56 (14): 5871-5879. 2008
- Kuksis A. 1992. Yolk lipids. Biochim Biophys Acta 1992 ; 1124 : 205-222
- Sharma SC. Mathematical modeling of the viscoelastic characteristics of egg yolk. J Food Sci 44:1123-1128, 1979
- Tsutsui T. Functional properties of heat-treated egg yolk low density lipoprotein. J Food Sci 53:1103- 1106, 1988
- Mizutani R, Nakamura R. The contribution of polypeptide moiety on the emulsifying properties of egg yolk low density lipoprotein (LDL). Lebens Wissen Technologie 18:60-63, 1985
- Martinet V, Beaumal V, Dalagalarrondo M, Anton M. Emulsifying properties and adsorption behavior of egg yolk lipoproteins (LDL and HDL) in o/w emulsions. Recent Research Dev Agric Food Chem,37: 103-116, 2002
- Dauphas S, Beaumal V, Gunning P, Mackie A, Wilde P, Vie V, Riaublanc A, Anton M. Structure modification in hen egg yolk low density lipoproteins layers between 30 and 45 mN/m observed by AFM. Colloids Surf B: Biointerfaces, 54 (2): 241-248, 2007
- Dauphas S, Beaumal V, Gunning P, Mackie A, Wilde P, Vié V, Riaublanc A, Anton M. Structures and rheological properties of hen egg yolk low density lipoproteins layers spread at the air-water interface at pH 3 and 7. Colloids Surf B: Biointerfaces, 57: 124-133, 2007
- Garland, Ph. D. dissertation, Studies on Egg Yolk Myelin Figures and Granule Low Density Lipoproteins, University Of British Columbia, 1973
- Shenton, Ph. D. dissertation, Membrane Composition and Performance of Food Emulsion, University of London, 1979
- Kiosseoglou VD. Egg yolk. In: Charalambous G, Doxastakis G, eds. Food Emulsifiers: Chemistry, Technology, Functional Properties and Applications. London: Elsevier, pp. 63-85, 1989
- Sirvente H. DEA, Mécanisme d’adsorption des LDL du jaune d’oeuf, 2003 20. Dauphas S, Beaumal V, Riaublanc A, Anton M. Hen egg yolk low density lipoproteins film spreading at the air-water and oil-water interfaces. J Agric Food Chem, 54 (10): 3733-3737, 2006
Biography
Dr. Marc Anton is Research Director at the Unit of Biopolymers Interactions Assemblies (BIA) belonging to the National Institute of Agronomic Research (INRA) of Nantes (France) since 1996. He had first dedicated its research on the functional properties of the hen egg yolk constituents. He is head of the Interfaces and Dispersed Systems (ISD) Team of the BIA Unit. Dr. Anton’s research interests include now proteins, lipids and lipoproteins structure and functionality (coming from animal or plant sources), protein aggregation, fabrication and characterisation of emulsions, colloidal interactions and interfacial properties. He is concerned by the construction and the stabilisation of dispersed systems by biopolymer assemblies, but also by their deconstruction under constraints miming gastro-intestinal tract. Dr. Anton published over 80 peer-reviewed scientific articles, three books, 20 book chapters, four patents and numerous conference proceedings.




Very good and informative article.